Electrolyte additive based on glycerol carbonate compounds and lithium ion battery
An electrolyte additive, glycerol carbonate technology, applied in the field of lithium batteries, can solve problems such as stability decline, achieve the effects of improving stability and uniformity, promoting rapid film formation, and improving battery performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
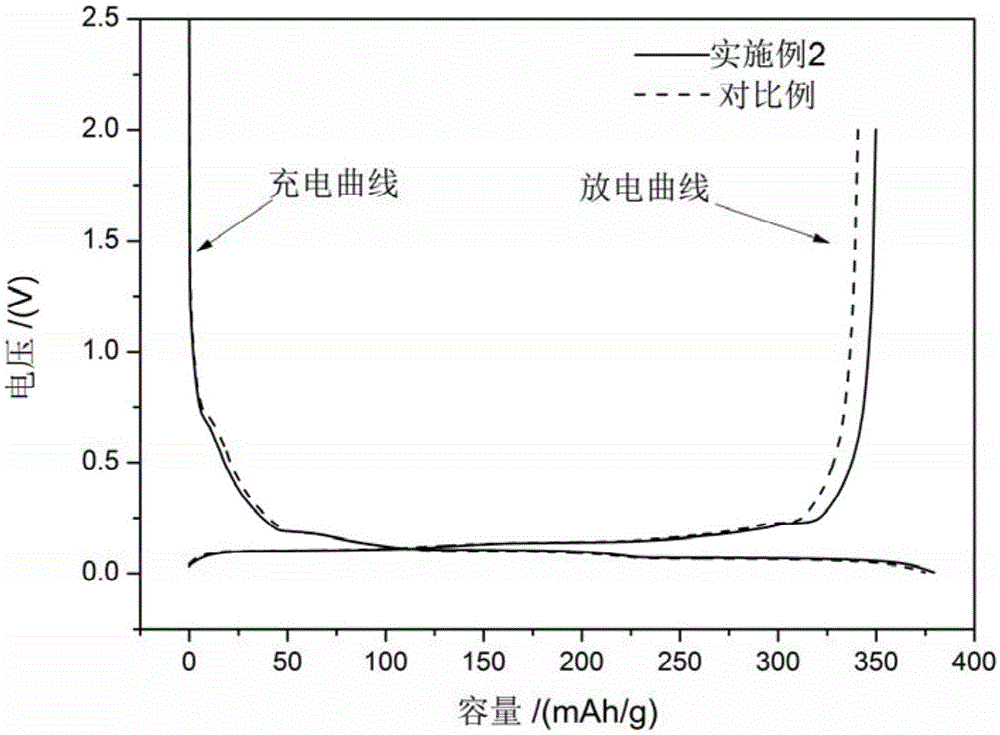
Image
Examples
Embodiment 1
[0038] Electrolyte preparation:
[0039] (1) The organic solvent is formulated into a mixed solvent according to the volume ratio of 50 parts of ethylene carbonate and 50 parts of dimethyl carbonate. Molecular sieves and calcium hydride are used to remove water to make the moisture lower than 10 ppm.
[0040] (2) The conductive lithium salt LiPF 6 Dissolve in the mixed solvent obtained in step (1), stir evenly, and make common electrolyte solution, wherein conductive lithium salt LiPF 6 The final concentration in common electrolyte is 1.0mol / L.
[0041] (3) Add tris(glycerol carbonate) borate additive whose mass is 0.5% of the total mass of lithium salt and organic solvent to the ordinary electrolyte prepared in step (2).
[0042] How to make the negative half-cell:
[0043] The mesophase carbon microsphere material, polyvinylidene fluoride (PVDF), and conductive graphite were weighed according to the mass ratio of 90:5:5, and put into a vacuum drying oven for drying treatm...
Embodiment 2
[0049] Electrolyte preparation:
[0050] (1) The organic solvent is formulated into a mixed solvent according to the volume ratio of 50 parts of ethylene carbonate and 50 parts of dimethyl carbonate. Molecular sieves and calcium hydride are used to remove water to make the moisture lower than 10 ppm.
[0051] (2) The conductive lithium salt LiPF 6 Dissolve in the mixed solvent obtained in step (1), stir evenly, and make common electrolyte solution, wherein conductive lithium salt LiPF 6 The final concentration in common electrolyte is 1.0mol / L.
[0052] (3) Add tris(glycerol carbonate) borate additive whose mass is 1.0% of the total mass of lithium salt and organic solvent to the ordinary electrolyte prepared in step (2).
[0053] The production and testing of the battery are the same as in Example 1.
Embodiment 3
[0055] (1) The organic solvent is formulated into a mixed solvent according to the volume ratio of 50 parts of ethylene carbonate and 50 parts of dimethyl carbonate. Molecular sieves and calcium hydride are used to remove water to make the moisture lower than 10 ppm.
[0056] (2) The conductive lithium salt LiPF 6 Dissolve in the mixed solvent obtained in step (1), stir evenly, and make common electrolyte solution, wherein conductive lithium salt LiPF 6 The final concentration in common electrolyte is 1.0mol / L.
[0057] (3) Add a tris(glycerol carbonate) borate additive whose mass is 2% of the total mass of the lithium salt and the organic solvent to the ordinary electrolyte prepared in step (2).
[0058] The production and testing of the battery are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com