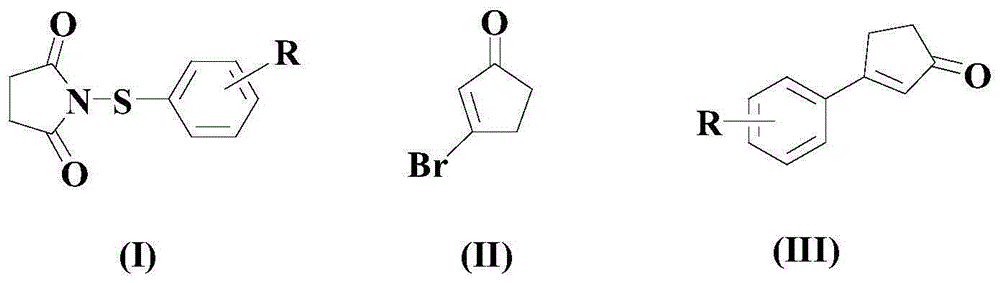Method for synthesizing aryl substituted cyclo-ketene compound
A synthesis method and compound technology are applied in the synthesis of ketene compounds and the synthesis field of aryl-substituted cyclic ketene compounds, which can solve the problem of high electron cloud density of aryl groups, achieve high yield, good application prospects and research potential effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]
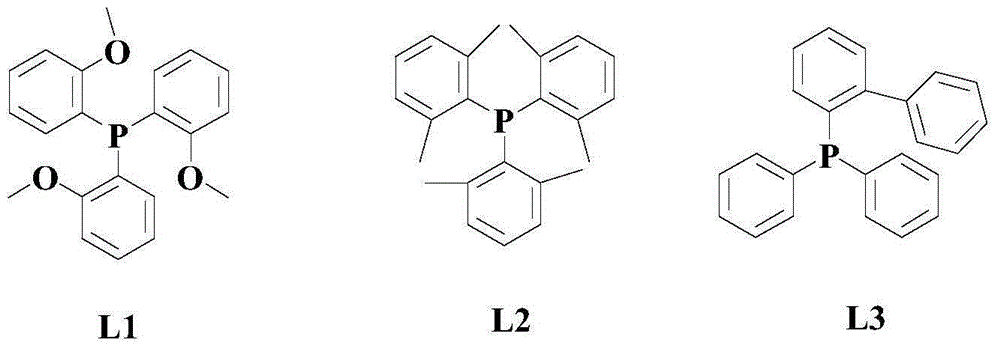
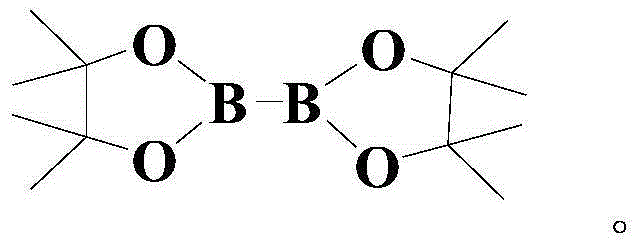
[0041] At room temperature and under a nitrogen atmosphere, add 100 mmol of the above formula (I) compound, 150 mmol of the above formula (II) compound, 4 mmol of the catalyst Pd (dpbpf)Cl 2 , 50mmol trifluoroacetic acid, 4mmol phosphine ligand L1 and 10mmol of the above additives, then warming up to 50°C, and stirring and reacting at this temperature for 8 hours;
[0042] After the reaction was completed, the reaction system was naturally cooled to room temperature, filtered, and the pH value of the filtrate was adjusted to 6-7 with a 10% NaOH aqueous solution with a mass percentage concentration, then deionized water was added, fully oscillated, and then extracted with ethyl acetate for 2 -3 times, the organic phases were combined, dried with anhydrous magnesium sulfate, concentrated under reduced pressure, and the residue was separated by 300-400 mesh silica gel column chromatography, and the mixture of petroleum ether and acetone with a volume ratio of 1:2 was ...
Embodiment 2
[0046]
[0047] At room temperature and under a nitrogen atmosphere, add 100 mmol of the above formula (I) compound, 200 mmol of the above formula (II) compound, 6 mmol of the catalyst Pd (dpbpf)Cl 2 , 75mmol trifluoroacetic acid, 6mmol phosphine ligand L1 and 15mmol of the above additives, then warming up to 60°C, and stirring and reacting at this temperature for 6 hours;
[0048] After the reaction was completed, the reaction system was naturally cooled to room temperature, filtered, and the pH value of the filtrate was adjusted to 6-7 with a 10% NaOH aqueous solution with a mass percentage concentration, then deionized water was added, fully oscillated, and then extracted with ethyl acetate for 2 -3 times, the organic phases were combined, dried with anhydrous magnesium sulfate, concentrated under reduced pressure, and the residue was separated by 300-400 mesh silica gel column chromatography, and the mixture of petroleum ether and acetone with a volume ratio of 1:2 was ...
Embodiment 3
[0052] The reaction formula is the same as in Example 1, and the specific operations are as follows: at room temperature and under a nitrogen atmosphere, add 100 mmol of the above formula (I) compound, 250mmol formula (II) compound, 8mmol catalyst Pd(dpbpf)Cl 2 , 100mmol trifluoroacetic acid, 8mmol phosphine ligand L1 and 20mmol of the above additives, then warming up to 70°C, and stirring and reacting at this temperature for 4 hours;
[0053] After the reaction was completed, the reaction system was naturally cooled to room temperature, filtered, and the pH value of the filtrate was adjusted to 6-7 with a 10% NaOH aqueous solution with a mass percentage concentration, then deionized water was added, fully oscillated, and then extracted with ethyl acetate for 2 -3 times, the organic phases were combined, dried with anhydrous magnesium sulfate, concentrated under reduced pressure, and the residue was separated by 300-400 mesh silica gel column chromatography, and the mixture of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com