Anticancer agent free of adverse reactions
A cancer, dose technology, applied in the field of pharmaceutical compositions for the treatment of cancer, which can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093]
[0094] 1. Production of Azido-Linker-SN-38
[0095]
[0096] SN38 (1.00 g, 2.55 mmol; Haorui) was suspended in 10 mL of anhydrous pyridine, then concentrated to dryness under vacuum at 50°C. Repeat this step with 10 mL of anhydrous THF. in N 2Under atmosphere, the resulting pale yellow solid was dissolved in 50 mL of anhydrous THF and 50 mL of anhydrous DMF, then cooled on ice. A 1.0 M solution of potassium tert-butoxide in THF (2.55 mL, 2.55 mmol) was added, forming an initial dark green color which became a thick orange suspension. After 15 min, 7-azido-1-cyano-2-hexyl N-(chloromethyl)-4-(N,N-diethylformamide)-phenylcarbamate (7.5 mL, 2.8 mmol) in THF. After 15 min at 4°C, the light orange mixture was allowed to warm to room temperature. After 1 hr, HPLC analysis (5 μL sample + 1 mL of CAN / TFA) indicated product / SN38=86 / 14. The pale yellow mixture was diluted with 200 mL of ethyl acetate, washed 2x100 mL of water, 100 mL of saturated aqueous NaCl, and dis...
Embodiment 2
[0108]
[0109] 1. Test conditions
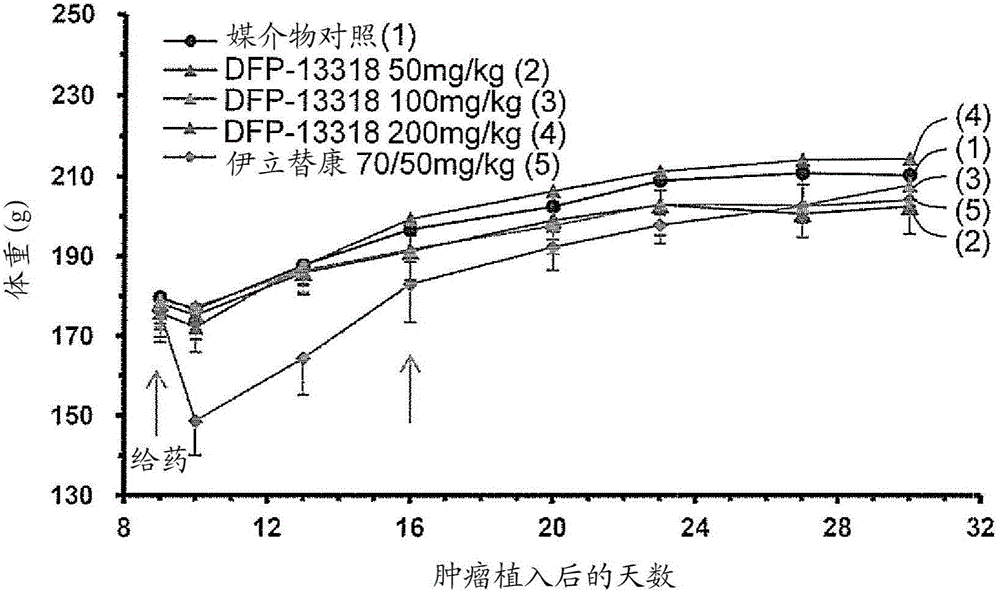
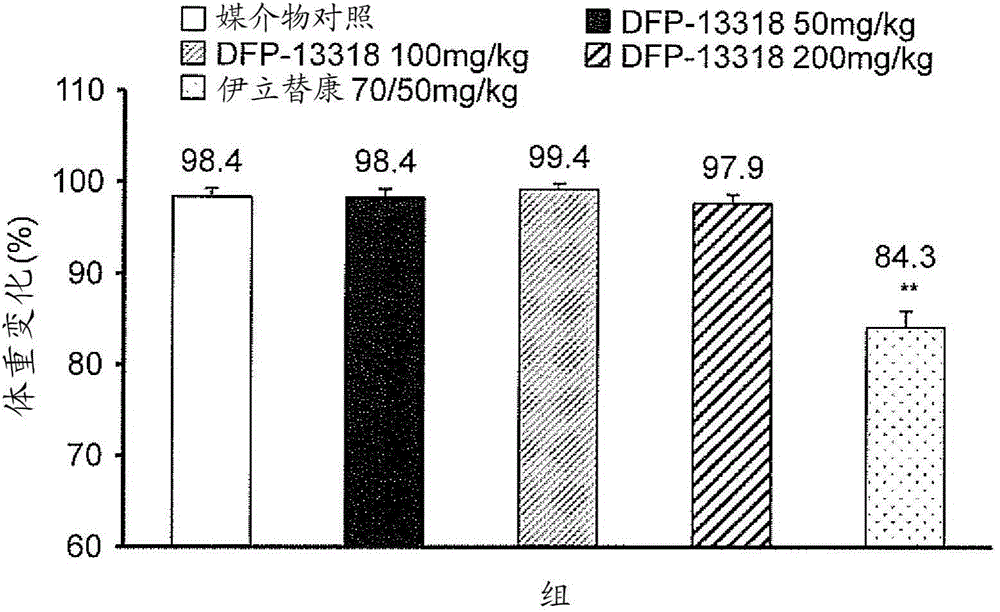
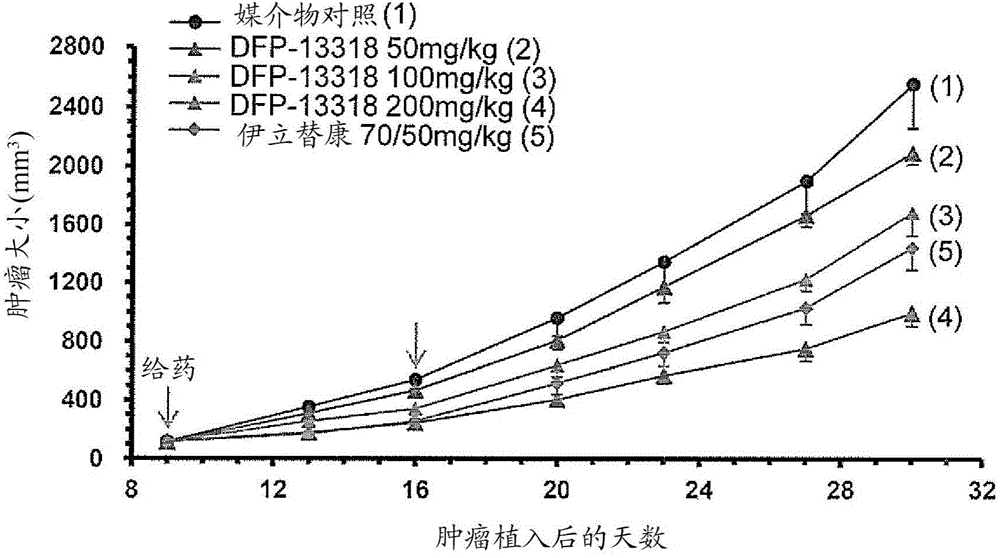
[0110] Toxicity, pharmacokinetic and efficacy studies were performed by intravenously injecting (iv) each test sample into tumor-bearing rats under the conditions shown in Tables 1 and 2.
[0111] [Table 1]
[0112] Rat Groups and Treatments Used for Efficacy Studies
[0113] Group
n*
treat
Dose (mg / kg)**
Pathway / Schedule
1
5
--
iv, qwk x 2 weeks
2
5
DFP-13318
50
iv, qwk x 2 weeks
3
5
DFP-13318
100
iv, qwk x 2 weeks
4
5
DFP-13318
200
iv, qwk x 2 weeks
5
5
irinotecan
70 / 50
iv, qwk x 2 weeks
[0114] Notes: *n: Number of animals, **Dose: Body weight adjusted (10 μL / g), ***Vehicle control: 10 mM acetate buffer, pH 5
[0115] [Table 2]
[0116] Auxiliary group for pharmacokinetic (PK) evaluation
[0117] Group
n*
treat
Dose (mg / kg)**
Pathway / Sched...
Embodiment 3
[0221]
[0222] This example was carried out to demonstrate that after intravenous administration of DFP-13318 for PK / PD (pharmacokinetics / pharmacodynamics) studies, DFP-13318 and its metabolite SN-38 were 29 were accumulated in tumor tissue collected from a tumor-bearing nude rat model.
[0223] In the PK / PD study, as shown in Table 15, 25 nude rats were used.
[0224] [Table 15]
[0225] Rat groups and treatments
[0226] Group
n*
treat
Dose (mg / kg)**
Pathway / Schedule
1
5
--
iv, qwk x 2 weeks
2
5
DFP-13318
50
iv, qwk x 2 weeks
3
5
DFP-13318
100
iv, qwk x 2 weeks
4
5
DFP-13318
200
iv, qwk x 2 weeks
5
5
irinotecan
70 / 50
iv, qwk x 2 weeks
[0227] Notes: *n: number of animals, **dose: body weight adjusted (10 μL / g), ***vehicle control: 10 mM acetate buffer, pH 5.
[0228] Twenty-five tumor fragments (with 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com