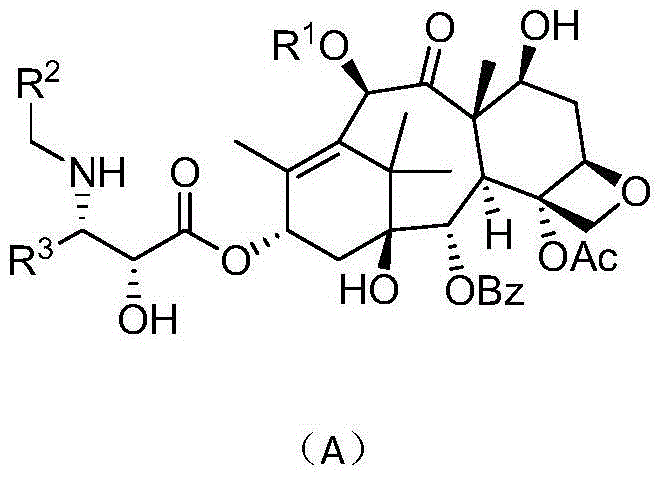
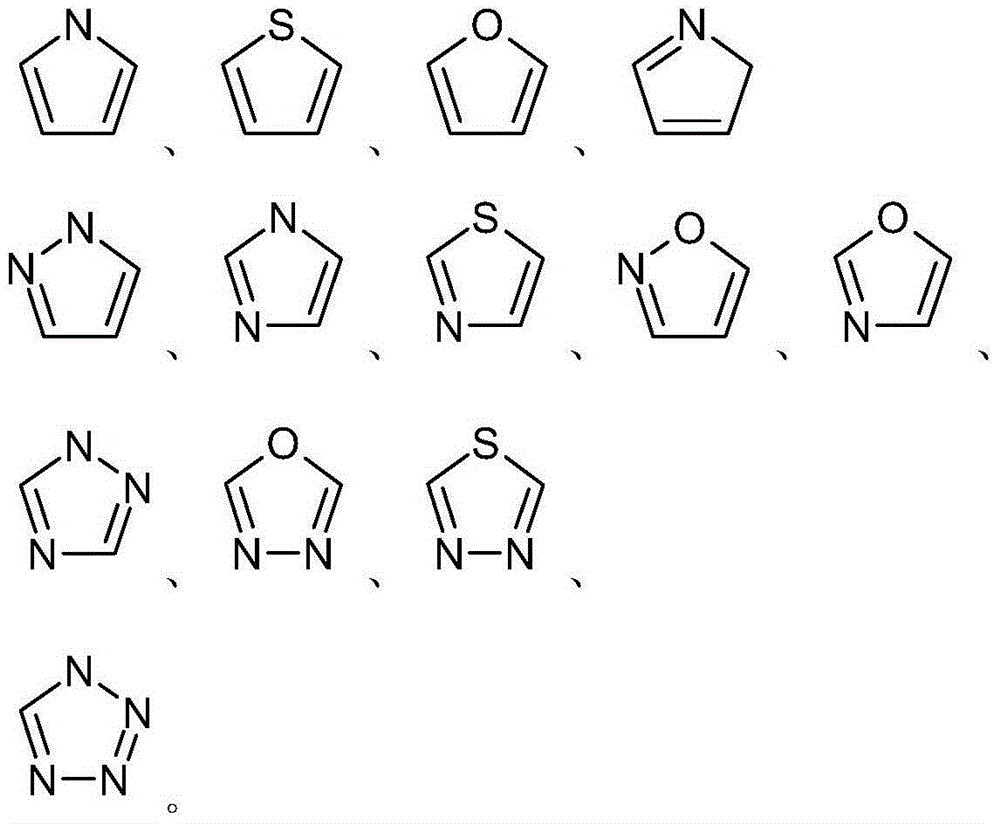
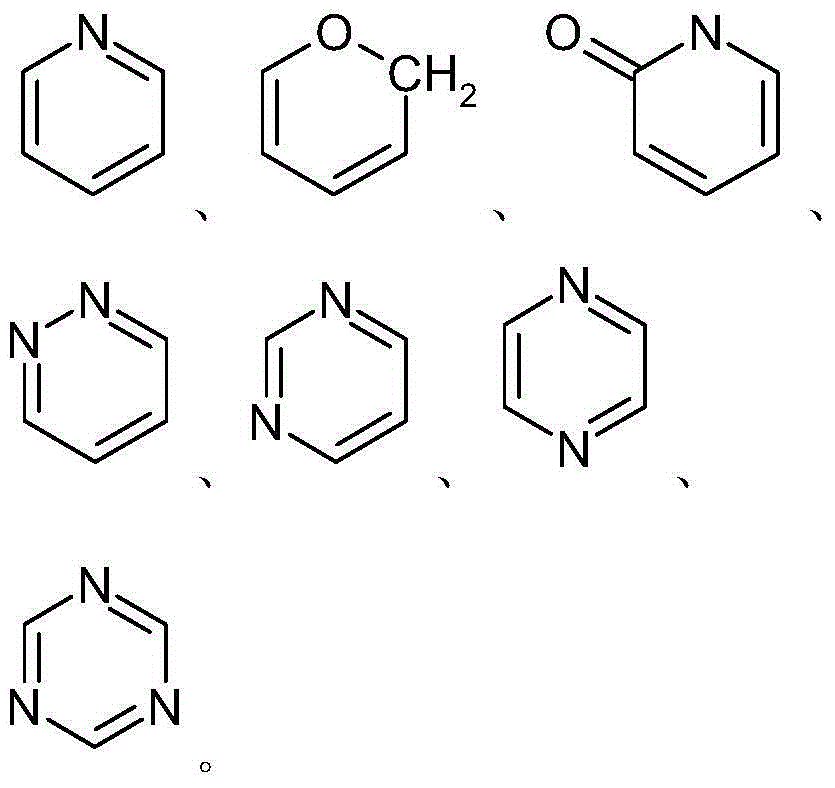
Derivatives of taxol and taxotere and composition and anti-tumor application of derivatives
A technology of paclitaxel derivatives and compounds, applied in the application field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Example 1 Preparation of 13-[(2'R,3'S)-3'-phenyl-2'-hydroxyl-3'-(thiophene-2-methyl)amino-propionyl]-10-deacetyl- Baccatin III (Ia, R 1 =H,R 2 = Thiophen-2-yl, R 3 =Ph)
[0105]
[0106] step 1
[0107] Add 211 mg (0.2 mmol) of compound III, 19 mg (0.08 mmol) of camphorsulfonic acid, 90 mg (0.8 mmol) of thiophene-2-carbaldehyde and 100 mg of molecular sieves into a dry reaction bottle, seal it, exchange argon and protect it with an argon balloon, and finally add 3 mL of no Water and dichloromethane were reacted for 4 hours under stirring at room temperature. 10 mg (0.16 mmol) of sodium cyanoborohydride was added, and the reaction was continued for 1 hour, and the starting point disappeared as detected by TLC. The reaction solution was filtered, and the filtrate was washed successively with saturated sodium bicarbonate and saturated brine, and dried over anhydrous sodium sulfate. It was filtered, concentrated, and separated by a silica gel column (petroleum eth...
Embodiment 2
[0111] Example 2 Preparation of 13-[(2'R,3'S)-3'-phenyl-2'-hydroxy-3'-(thiophene-3-methyl)amino-propionyl]-10-deacetyl- Baccatin III (Ib, R 1 =H,R 2 = Thiophen-3-yl, R 3 =Ph)
[0112]
[0113] step 1
[0114] Add compound III 400mg (0.378mmol), camphorsulfonic acid 35mg (0.15mmol), thiophene-3-carboxaldehyde 102mg (0.9mmol) and molecular sieve 100mg in dry reaction bottle, seal, exchange argon and protect with argon balloon, finally add 4mL without Water and dichloromethane were reacted for 4 hours under stirring at room temperature. 19 mg (0.30 mmol) of sodium cyanoborohydride was added, and the reaction was continued for 1 hour, and the starting point disappeared as detected by TLC. The reaction solution was filtered, and the filtrate was washed successively with saturated sodium bicarbonate and saturated brine, and dried over anhydrous sodium sulfate. It was filtered, concentrated, and separated by a silica gel column (petroleum ether: acetone = 5:1) to obtain 184...
Embodiment 3
[0118] Example 3 Preparation of 13-[(2'R,3'S)-3'-phenyl-2'-hydroxyl-3'-(thiazole-4-methyl)amino-propionyl]-10-deacetyl- Baccatin III (Ic, R 1 =H,R 2 = Thiazol-4-yl, R 3 =Ph)
[0119]
[0120] step 1
[0121] Add 500 mg (0.47 mmol) of compound III, 45 mg (0.19 mmol) of camphorsulfonic acid, 130 mg (1.15 mmol) of thiazole-4-carbaldehyde and 100 mg of molecular sieve into a dry reaction bottle, seal it, exchange argon and protect it with an argon balloon, and finally add 5 mL of Water and dichloromethane were reacted for 4 hours under stirring at room temperature. 25 mg (0.4 mmol) of sodium cyanoborohydride was added, and the reaction was continued for 1 hour, and the starting point disappeared as detected by TLC. The reaction solution was filtered, and the filtrate was washed successively with saturated sodium bicarbonate and saturated brine, and dried over anhydrous sodium sulfate. It was filtered, concentrated, and separated by a silica gel column (petroleum ether: a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com