Copper electrolyte purifying technology
A copper electrolyte and purification process technology, which is applied in the separation of impurities and copper electrolyte purification process, can solve the problems of poor working environment, increased electrolyte resistance, and low direct copper recovery rate, and overcome the problem of low purification efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
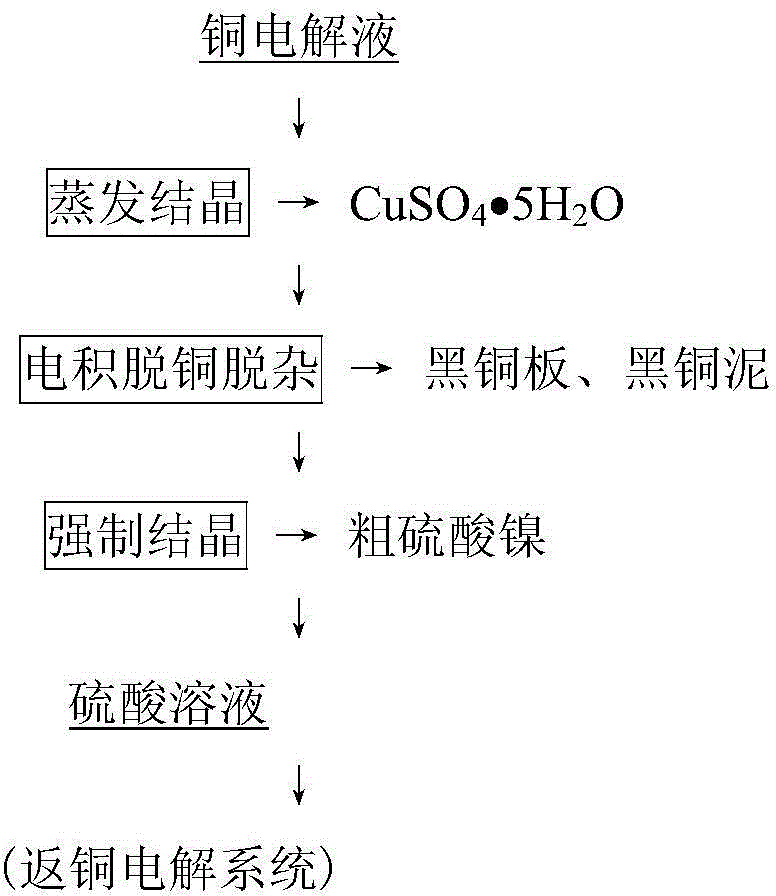
[0045] Take 1L of copper electrolytic refining electrolyte, add Sb according to the molar ratio of Sb(III) / As(V) 2:1 2 o 3 , and add hydrated Sb according to Sb(IV) / Sb(III) molar ratio 1:7 2 o 4 As an adsorbent, it was stirred and adsorbed at 75°C for 1 hour and then filtered to obtain the loaded adsorbent and the adsorbed liquid. The obtained loaded adsorbent was mixed with water at a solid-to-liquid ratio of 1:6 g / ml, then added with sodium carbonate to adjust the pH to 10.6, stirred and desorbed at 70°C for 3 hours, and filtered to obtain the oxide of antimony hydrate and the desorbed solution. After the desorption solution was stirred and cooled at 10°C for 1 hour, the sodium arsenate product and its crystallized liquid were obtained by filtration. After the obtained adsorbed liquid is pre-decoppered by cooling and crystallizing copper sulfate at 5°C, ammonium sulfate is added according to 1.5 times the theoretical amount of ammonium sulfate double salt formed by Cu and...
Embodiment 2
[0048] Take 5m of the primary decopper solution produced in the copper electrolyte purification process 3 According to 1 times the theoretical amount of ammonium sulfate double salt formed by Cu and Ni, ammonia gas was introduced, stirred at -18°C for 6 hours, and the mixed crystal of copper nickel ammonium sulfate double salt and the solution after nickel removal were obtained by filtration. After the obtained nickel-removed liquid is adsorbed and removed, Sb is added according to the molar ratio of (Sb(III)+Bi(III)) / As(V) 2.5:1 2 o 3 and Bi 2 o 3 The mixture is used as the adsorbent, the Sb in the mixture 2 o 3 with Bi 2 o 3 The molar ratio of Sb(V) / Sb(III) is 4:1, and the hydrated Sb is added according to the Sb(V) / Sb(III) molar ratio of 1:5 2 o 5 , stirred and adsorbed at 85°C for 1.5h, and filtered to obtain the loaded adsorbent and the adsorbed liquid. The obtained adsorbed liquid is returned to the copper electrolysis production system for use; the obtained cop...
Embodiment 3
[0052] Take 1m of the secondary decopper solution produced in the copper electrolyte purification process 3 , according to wherein Cu and Ni form 0.8 times of the theoretical amount of ammonium sulfate double salt, add the nickel ammonium sulfate crystallization solution obtained in Example 2, stir at -5°C for 20h, filter to obtain copper nickel ammonium sulfate double salt mixed crystals and crystallization thereof liquid. The obtained liquid after crystallization is returned to the copper electrolysis production system for use; the obtained copper-nickel ammonium sulfate double salt mixed crystal is added with water at a solid / liquid ratio of 1:5g / ml, stirred and dissolved at 60°C; the obtained dissolved liquid is used as the organic phase selectivity of the P204-kerosene system Extract copper, the concentration of P204 in the organic phase is 10%, load the organic phase with 2mol / L H 4 SO 4 solution as back-extraction. The back-extraction liquid is concentrated to crysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com