Method for synthesizing 2-halogenated nicotinic acid ester and intermediates thereof through microwave method
A technology of halogenated nicotinic acid esters and intermediates, which is applied in the field of microwave synthesis of 2-halogenated nicotinic acid esters and its intermediates, which can solve the problems of intractable, difficult, and multi-treatment waste liquids, and achieve low synthesis energy consumption , short reaction time, avoid flammable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
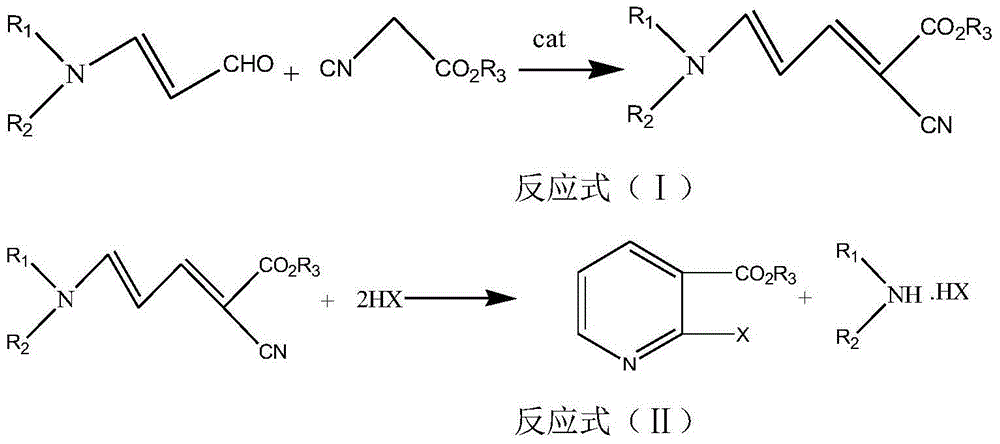
[0034] Example 1 Synthesis of methyl 2-chloronicotinate
[0035] In a 500mL three-necked flask equipped with a thermometer, first add 69mL (0.5mol) of N,N-dibutylaminoacrolein and KHCO 3 5.0 g, and then add 65 mL (0.6 mol) of methyl cyanoacetate, and put the prepared device into a microwave apparatus. Set microwave radiation conditions, react under the conditions of temperature 60°C, microwave power 100W and frequency 915MHz, TLC detection (petroleum ether: dichloromethane 1:2, sublimation iodine color development) N,N-dibutylamine The reaction of acrolein is complete. Afterwards, HCl gas was introduced again, and the microwave irradiation conditions were the same as above, and the reaction was carried out for about 30 minutes, and the reaction was tracked by HPLC until the reaction was completed. Then after the reaction, add sodium carbonate solution to adjust the pH=5-6, separate the layers, extract the aqueous layer with ethyl acetate 20mL×3 times, combine the organic lay...
Embodiment 2
[0044] Example 2 Synthesis of methyl 2-bromonicotinate
[0045] In a 500mL three-neck flask equipped with a thermometer, first add 61mL (0.5mol) of 3-diethylaminoacrolein and 20mL of diethanolamine, then add 65mL (0.6mol) of methyl cyanoacetate, and put the prepared device into the microwave instrument. Set up the microwave radiation conditions, react at a temperature of 60°C, a microwave power of 200W, and a frequency of 915MHz, TLC detection (petroleum ether: dichloromethane 1:2, sublimation iodine color development) 3-diethylaminopropene Aldehydes react completely. Afterwards, HBr gas was introduced, and the microwave irradiation conditions were the same as above, and the reaction was performed for about 35 minutes, and the reaction was tracked by HPLC until the reaction was completed. Then after the reaction is finished, add a mass fraction of 10% potassium hydroxide solution to adjust the pH=5-6, separate the layers, extract the aqueous layer with 20 mL of ethyl acetate...
Embodiment 3
[0047] Example 3 Synthesis of ethyl 2-chloronicotinate
[0048] In a 500mL three-necked flask equipped with a thermometer, first add 62mL (0.5mol) of 3-dimethylaminoacrolein and 10mL of piperidine, then add 65mL (0.6mol) of ethyl cyanoacetate, and put the prepared device into the microwave instrument. Set microwave radiation conditions, react under the conditions of temperature 120°C, microwave power 350W and frequency 2450MHz, TLC detection (petroleum ether: dichloromethane 1:2, sublimation iodine color development) 3-dimethylaminoacrolein The response is complete. Afterwards, HCl gas was introduced again, the microwave irradiation conditions were as above, and the reaction was tracked by HPLC until the reaction was completed. Then after the reaction is over, add potassium carbonate solution to adjust the pH=5-6, separate the layers, extract the aqueous layer with 20 mL of dichloromethane×3 times, combine the organic layers, add anhydrous Na 2 SO 4 Dry, filter, evaporate ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com