Preparation method of zinc oxide nanoparticles for rapid detection of explosive atmosphere
A technology of zinc oxide nano and explosives, applied in the field of gas-sensitive sensing materials and functional materials science, can solve the problems of high power consumption, unfavorable detection, high operating temperature, etc., achieve low detection limit, fast response, and improve adsorption performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
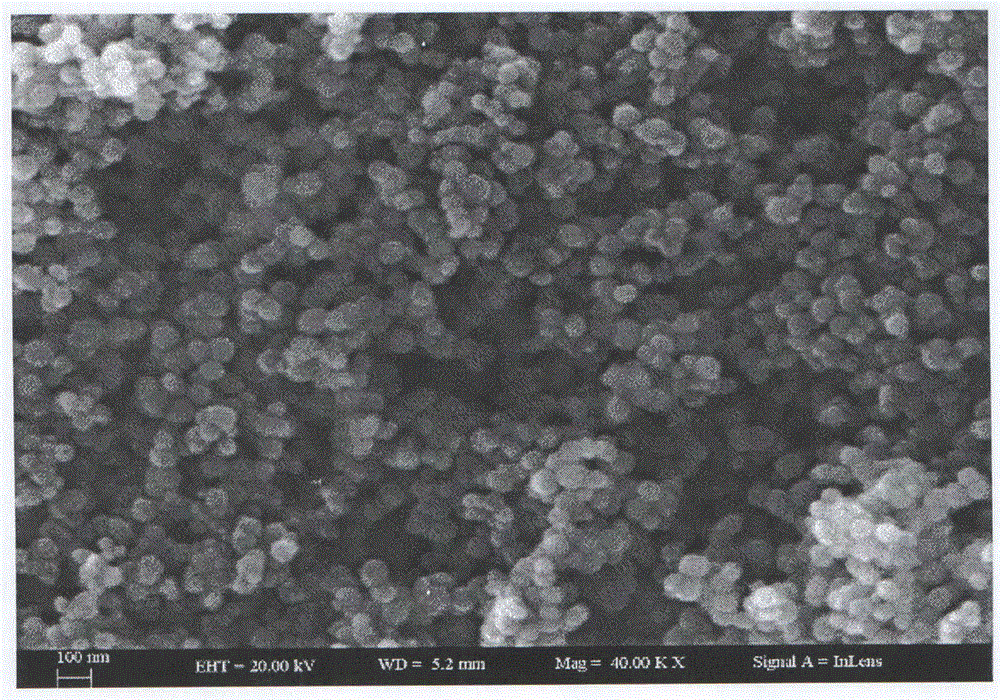
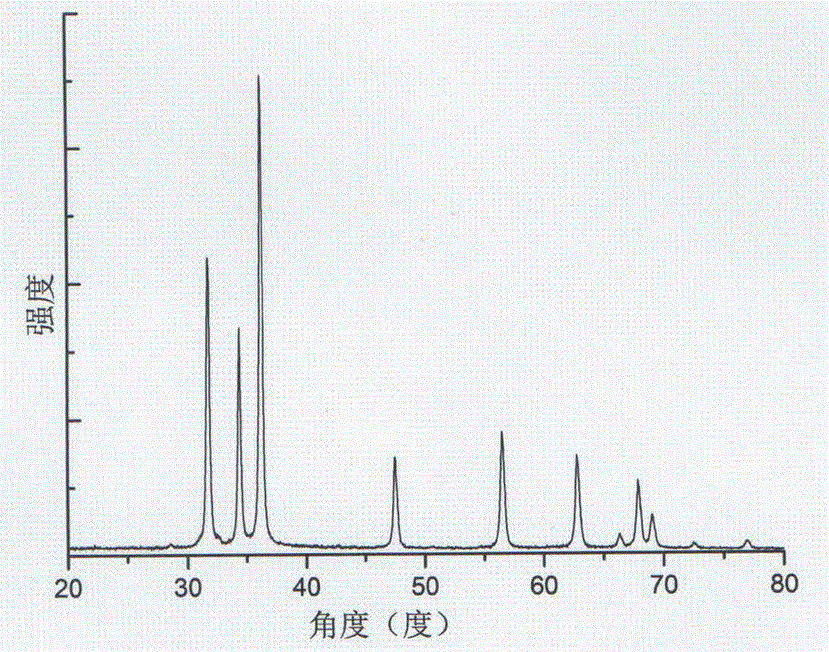
Image
Examples
Embodiment 1
[0038] a. Dissolve 1.4875 grams of zinc nitrate hexahydrate in blue bottle A containing 50 ml of absolute ethanol;
[0039] b. Dissolve 0.4208 g of potassium hydroxide in blue-necked bottle B containing 50 ml of absolute ethanol, stir magnetically until completely dissolved, then transfer the blue-necked bottle A of step a and the blue-necked bottle B of step b to the refrigerator , frozen for 12 hours;
[0040] c. Add the solution in the blue-necked bottle B to the blue-necked bottle A, stir magnetically until the mixture is uniform, put the solution in a water bath, control the temperature of the water bath to 60 °C, and stir for 24 hours to obtain an opaque colloid;
[0041]d, disperse the opaque colloid obtained in step c in n-heptane, centrifuge, disperse the centrifuged sediment in absolute ethanol, centrifuge again, and use two washings as a washing cycle, repeat the washing cycle 3 times, The washed precipitate was transferred to a watch glass, dried in an oven at 80 ...
Embodiment 2
[0044] a. Dissolve 1.4875 grams of zinc nitrate hexahydrate in blue bottle A containing 50 ml of absolute ethanol;
[0045] b. Dissolve 0.3000 g of sodium hydroxide in the blue-necked bottle B containing 50 ml of absolute ethanol, stir magnetically until completely dissolved, then transfer the blue-necked bottle A of step a and the blue-necked bottle B of step b to the refrigerator , frozen for 12 hours;
[0046] c. Add the solution in the blue-necked bottle B to the blue-necked bottle A, stir magnetically until evenly mixed, put the solution in a water bath, control the temperature of the water bath to 60°C, and stir for 24 hours to obtain an opaque colloid;
[0047] d, disperse the opaque colloid obtained in step c in n-heptane, centrifuge, disperse the centrifuged sediment in absolute ethanol, centrifuge again, and use two washings as a washing cycle, repeat the washing cycle 3 times, The washed precipitate was transferred to a watch glass, dried in an oven at 80 °C for 12...
Embodiment 3
[0050] a. Dissolve 1.4875 grams of zinc nitrate hexahydrate and 0.0727 grams of nickel nitrate in blue bottle A containing 50 ml of absolute ethanol;
[0051] b. Dissolve 0.3000 g of sodium hydroxide in the blue-necked bottle B containing 50 ml of absolute ethanol, stir magnetically until completely dissolved, then transfer the blue-necked bottle A of step a and the blue-necked bottle B of step b to the refrigerator , frozen for 12 hours;
[0052] c. Add the solution in the blue-necked bottle B to the blue-necked bottle A, stir magnetically until evenly mixed, put the solution in a water bath, control the temperature of the water bath to 25°C, and stir for 0.5 h to obtain an opaque colloid;
[0053] d. Disperse the opaque colloid obtained in step c in n-hexane, centrifuge, disperse the centrifuged sediment in absolute ethanol, centrifuge again, use two washes as a wash cycle, repeat the wash cycle 3 times, and The washed precipitate was transferred to a watch glass, dried in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com