Synthesis method of 2-arylbenzothiazole compound
A technology of benzothiazole and synthesis method, which is applied in the field of synthesis of 2-arylbenzothiazole compounds, can solve the problems of complex reaction system and high reaction temperature, and achieve the effects of easy-to-obtain raw materials, mild reaction conditions and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
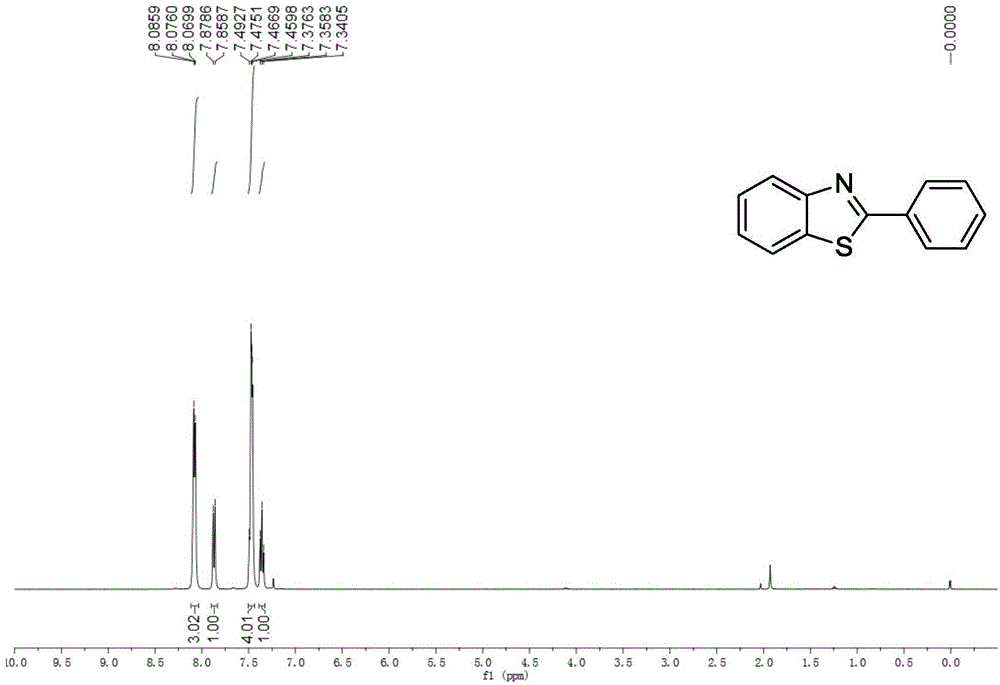
[0032] Embodiment 1: the synthesis of 2-phenylbenzothiazole
[0033]
[0034] Accurately measure the reaction substrate o-aminothiophenol (0.318mL, 3mmol), benzonitrile (0.306mL, 3mmol), and trifluoromethanesulfonic acid (3μL, 0.03mmol), and add them to a 25mL Schlenk bottle successively, Placed in an oil bath at 25°C for 14 hours. After stopping the reaction, extract with water and ethyl acetate as the extractant, and the extracted organic phase is washed with anhydrous Na 2 SO 4 After drying for 2 h and removing the solvent by rotary evaporation, the crude target product benzothiazole was obtained. This was followed by a recrystallization step: the crude benzothiazole was placed in a 50 mL flask. Put the flask into the heating mantle and heat it to 50°C, slowly add absolute ethanol dropwise until it is completely dissolved, then take the flask out of the heating mantle and let it cool down at room temperature for 4 hours, and filter it with filter paper after the white...
Embodiment 2
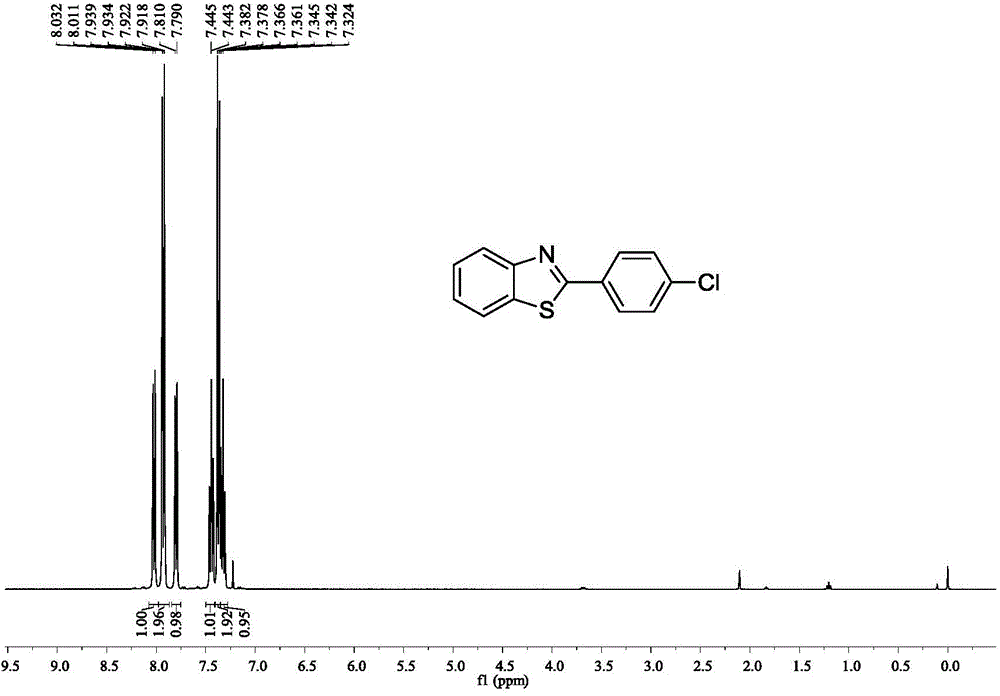
[0036] Embodiment 2: the synthesis of 2-(4-chloroaryl) benzothiazole
[0037]
[0038] Accurately measure the reaction substrate o-aminothiophenol (0.318mL, 3mmol), p-chlorobenzonitrile (0.412g, 3mmol), methanesulfonic acid (0.0028g, 0.03mmol), and add them to a 25mL Schlenk bottle in turn , placed in an oil bath at 40°C for 14 hours. After stopping the reaction, extract with water and ethyl acetate as the extractant, and the extracted organic phase is washed with anhydrous Na 2 SO 4 After drying for 2 h and removing the solvent by rotary evaporation, the crude target product 2-(4-chloroaryl)benzothiazole was obtained. This was followed by a recrystallization step: the crude 2-(4-chloroaryl)benzothiazole was placed in a 50 mL flask. Put the flask into the heating mantle and heat it to 50°C, slowly add absolute ethanol dropwise until it is completely dissolved, then take the flask out of the heating mantle and let it cool down at room temperature for 4 hours, and filter i...
Embodiment 3
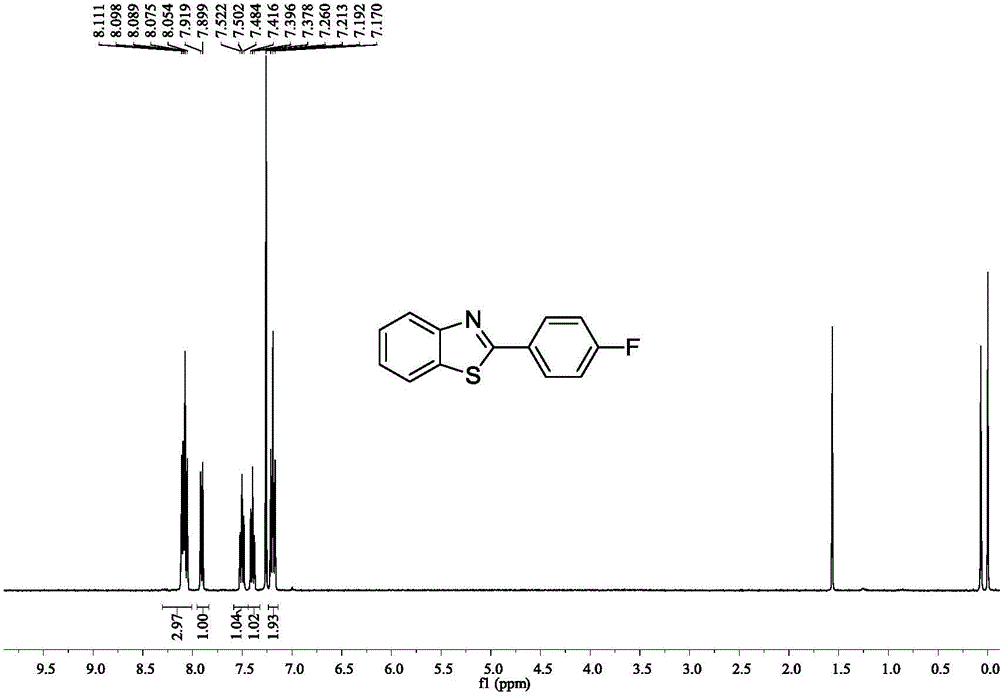
[0040] Example 3: Synthesis of 2-(4-fluoroaryl)benzothiazole
[0041]
[0042] Accurately measure the reaction substrate o-aminothiophenol (0.318mL, 3mmol), p-fluorobenzonitrile (0.363g, 3mmol), D-camphor-10-sulfonic acid (0.139g, 0.6mmol), add to In a 25mL Schlenk bottle, put it in an oil bath at 50°C for 14h. After stopping the reaction, extract with water and ethyl acetate as the extractant, and use anhydrous Na2 SO 4 After drying for 2 h and removing the solvent by rotary evaporation, the crude target product 2-(4-fluoroaryl)benzothiazole was obtained. This was followed by a recrystallization step: the crude 2-(4-fluoroaryl)benzothiazole was placed in a 50 mL flask. Put the flask into a heating mantle and heat it to 50°C, slowly add absolute ethanol dropwise until it is completely dissolved, then take out the flask from the heating mantle and let it cool down at room temperature for 4 hours, and filter it with filter paper after the white crystals are completely preci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com