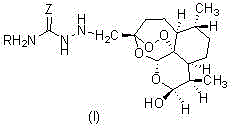
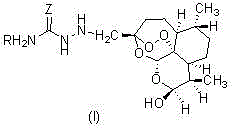
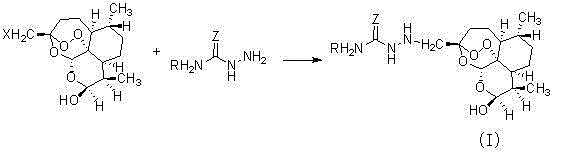Semicarbazide dihydroartemisinin derivative as well as preparation method and application of semicarbazide dihydroartemisinin derivative
A technology of semicarbazide dihydroartemisinin and derivatives, which is applied in the field of semicarbazide dihydroartemisinin derivatives and their preparation and application, and can solve the problems of less research on the modification of dihydroartemisinin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-(aminothiocarbazido)methylene-6,9-dimethyl-3,12-oxo-12H - Preparation of pyrano[4,3-j]-1,2-benzodithiapine-10(3H)alcohol (1)
[0024] The structure of compound (1) is shown below:
[0025]
[0026] Add 3.62 g (0.01 mol) of (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-bromomethylene-6,9-dimethyl-3,12 to the dry reactor -Oxo-12H-pyrano[4,3-j]-1,2-benzodithiapine-10(3H)alcohol and 20mLTHF, stirred, added 1.58g (0.02mol) of pyridine and 1.09g (0.012 mol) thiosemicarbazide, reacted for 12 hours, and evaporated the solvent under reduced pressure. Add 20mL of ethyl acetate and 20mL of saturated sodium bicarbonate solution to the residue, stir, separate layers, extract 15mLX2 of the aqueous layer with ethyl acetate, combine the organic phases, dry, filter, concentrate, and purify by column chromatography to obtain the target compound (1), The yield is 55%.
Embodiment 2
[0028] (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-(carbahydrazino)methylene-6,9-dimethyl-3,12-oxo-12H-pyridine Fro[4,3-j]-1,2-benzodithiapine-10(3H)alcohol (2); the structure of compound (2) is shown below:
[0029]
[0030] Add 3.62 g (0.01 mol) of (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-bromomethylene-6,9-dimethyl-3,12 to the dry reactor -Oxo-12H-pyrano[4,3-j]-1,2-benzodithiapine-10(3H)alcohol and 22mLTHF, stirred, added 1.58g (0.02mol) of pyridine and 1.09g (0.012 mol) semicarbazide, reacted for 12 hours, and evaporated the solvent under reduced pressure. Add 20mL ethyl acetate and 20mL saturated sodium bicarbonate solution to the residue, stir, separate layers, extract 15mLX2 of the aqueous layer with ethyl acetate, combine the organic phases, dry, filter, concentrate, and purify by column chromatography to obtain 3-ureaaminodihydro Artemisinin, yield 52%.
Embodiment 3
[0032] (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-(methylcarbahydrazino)methylene-6,9-dimethyl-3,12-oxo-12H- Pyrano[4,3-j]-1,2-benzodithiapine-10(3H)ol (3);
[0033] The structure of compound (3) is shown below:
[0034]
[0035] Add 3.18 g (0.01 mol) of (3R, 5aS, 6R, 8aS, 9R, 12S, 12aR)-octahydro-3-chloromethylene-6,9-dimethyl-3,12 to the dry reactor -Oxo-12H-pyrano[4,3-j]-1,2-benzodithiapine-10(3H)alcohol and 20mL acetonitrile, stirred, added 1.58g (0.02mol) pyridine, 0.82 g (0.0005 mol) KI and 1.07g (0.012mol) methylcarbamohydrazide were reacted for 24 hours, and the solvent was distilled off under reduced pressure. Add 20mL ethyl acetate and 20mL saturated sodium bicarbonate solution to the residue, stir, separate layers, extract the aqueous layer with ethyl acetate 15mLX2, combine the organic phases, dry, filter, concentrate, and purify by column chromatography to obtain compound (3). The rate is 66%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com