Medicinal composition for treating complex infection and preparation method of medicinal composition
A combined infection and composition technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, antibacterial drugs, etc., can solve the problems of inconvenient clinical use, long treatment time, and pain of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
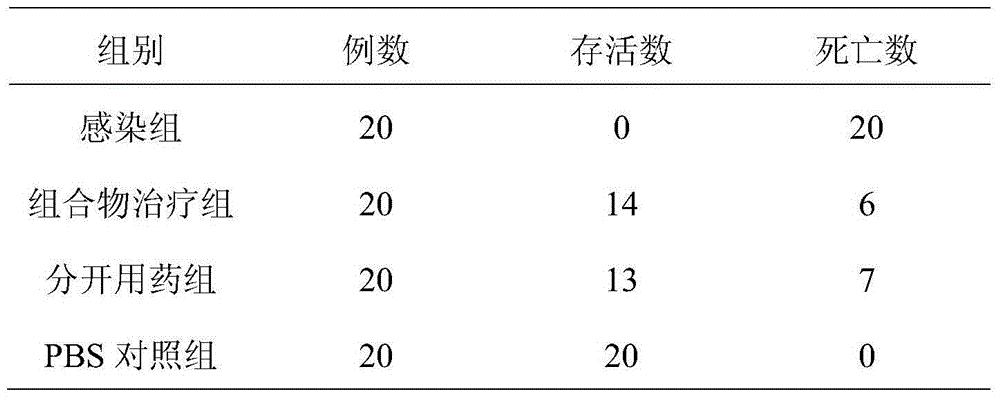
[0011] Telavancin hydrochloride 250g (calculated as telavancin), anidulungin 50g, mannitol 300g, hydrochloric acid buffer in proper amount.
[0012] The preparation process is as follows: accurately weigh the prescribed amount of telavancin hydrochloride and anidifungin, add an appropriate amount of water for injection to dissolve; add the prescribed amount of mannitol and hydrochloric acid buffer to the above solution in sequence, and adjust the pH to 5.0 , and then use a 0.1μm ultrafiltration membrane to remove the heat source, the filtrate is sterilized and filtered through a 0.22μm microporous membrane, add water for injection to make up to 15L, and then divide it into 1000 bottles, freeze-dry, press the stopper, roll the cap, and pass the inspection Labeled and packaged to obtain the powder for injection of the composition.
[0013] Investigate the therapeutic effect of the pharmaceutical composition lyophilized powder preparation prepared in Example 1 and the commerciall...
Embodiment 2
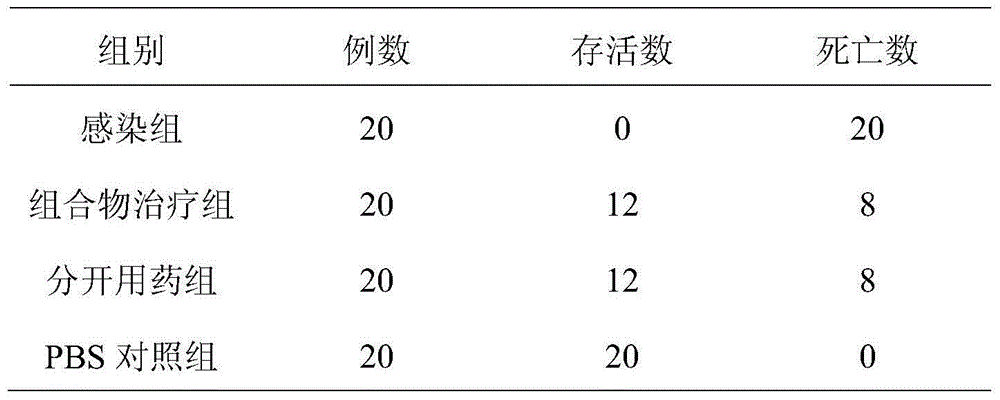
[0023] Vancomycin hydrochloride 100g (calculated as telavancin), amphotericin B 125g, mannitol 300g, appropriate amount of hydrochloric acid buffer.
[0024] The preparation process is as follows: accurately weigh the prescribed amount of vancomycin hydrochloride and amphotericin B, add an appropriate amount of water for injection to dissolve; add the prescribed amount of mannitol and hydrochloric acid buffer to the above solution in turn, adjust the pH to 5.0, Finally, use a 0.1 μm ultrafiltration membrane to remove the heat source, and the filtrate is sterilized and filtered through a 0.22 μm microporous membrane, and then added water for injection to make up to 25L, then divided into 1000 bottles, freeze-dried, plugged, and capped, and pasted after passing the inspection Sign the package to get the powder for injection of the composition.
[0025] Investigate the therapeutic effect of the pharmaceutical composition lyophilized powder preparation prepared in Example 2 and th...
Embodiment 3
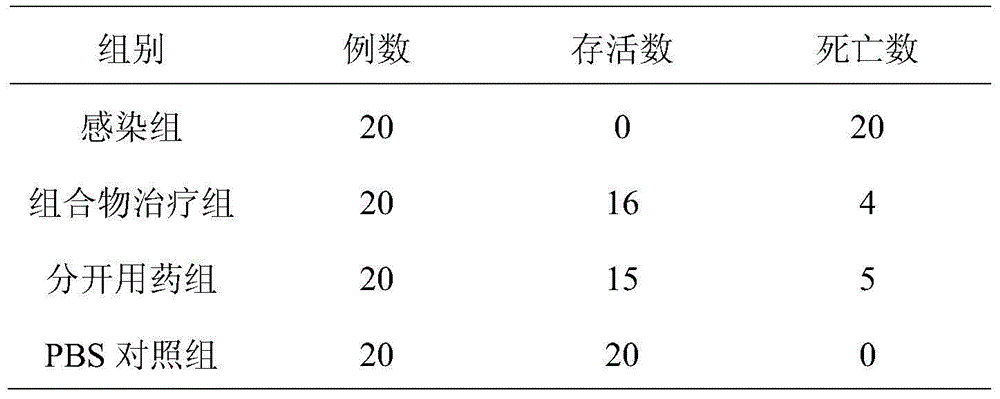
[0035] Dalbavancin hydrochloride 500g (calculated as dalbavancin), caspofungin acetate 250mg (calculated as caspofungin), lactose 150g, mannitol 150g, appropriate amount of tartrate buffer.
[0036] The preparation process is as follows: accurately weigh the prescribed amount of dalbavancin hydrochloride and caspofungin acetate, add an appropriate amount of water for injection to dissolve; sequentially add the prescribed amount of mannitol, lactose, and tartrate buffer to the above solution, adjust pH to 5.2, then use 0.1μm ultrafiltration membrane to remove heat source, the filtrate is sterilized and filtered through 0.22μm microporous membrane, add water for injection to make up to 25L, then divide into 1000 bottles, freeze-dry, press stopper, roll cap, After passing the inspection, the powder for injection of the composition is obtained by labeling and packaging.
[0037]Investigate the therapeutic effect of the pharmaceutical composition freeze-dried powder preparation pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com