Yellow long-afterglow luminescent material and preparation method thereof
A technology of long afterglow materials and luminescent materials, applied in the field of materials science, can solve the problems of long afterglow time, difficulty of yellow long afterglow luminescent materials, good chemical stability, etc., and achieve non-radiation, significant technological progress, and stable chemical properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
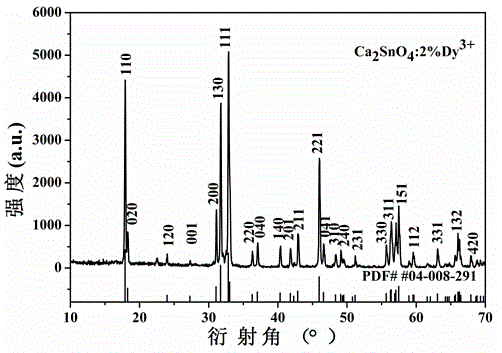
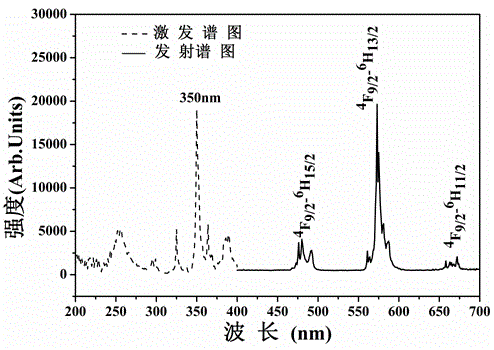
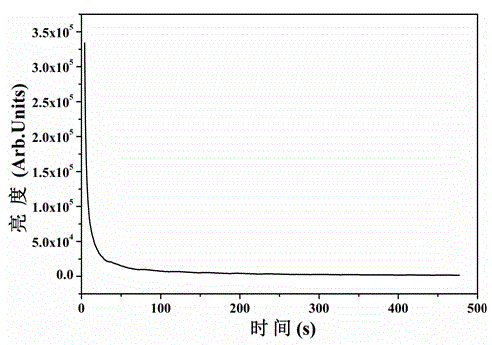
[0020] A yellow long-lasting luminescent material, using calcium stannate as the luminescent material matrix, using trivalent dysprosium ion Dy 3+ As a luminescent activator, boric acid was used as a flux for material synthesis.
[0021] The above-mentioned preparation method of a yellow long-lasting luminescent material specifically comprises the following steps:
[0022] 1) Calculated as a molar percentage, i.e. CaCO 3 : SnO 2 : Dy 2 o 3 :H 3 BO 3 The ratio of 65.463%: 32.814%: 0.082%: 1.641%, weigh chemically pure CaCO 3 , SnO 2 , Dy 2 o 3 and H 3 BO 3 ;
[0023] 2) Grind the above-mentioned raw materials in a mortar for 1 hour to mix the powder evenly, then pass through a 200-mesh sieve, and press the sieved powder into tablets;
[0024] 3) Put the above mixture into a crucible, then burn it in a high-temperature furnace at 1350°C for 4 hours, cool it down to room temperature naturally, take it out, and crush it through a 200-mesh sieve to obtain a yellow long...
Embodiment 2
[0030] A yellow long-lasting luminescent material, using calcium stannate as the luminescent material matrix, using trivalent dysprosium ion Dy 3+ As a luminescent activator, boric acid was used as a flux for material synthesis.
[0031] The above-mentioned preparation method of a yellow long-lasting luminescent material specifically comprises the following steps:
[0032] 1) Calculated as a molar percentage, i.e. CaCO 3 : SnO 2 : Dy2 o 3 :H 3 BO 3 65.383%: 32.841%: 0.164%: 1.642%, weigh chemically pure CaCO 3 , SnO 2 , Dy 2 o 3 and H 3 BO 3 ;
[0033] 2) Grind the above-mentioned raw materials in a mortar for 1 hour to mix the powder evenly, then pass through a 200-mesh sieve, and press the sieved powder into tablets;
[0034] 3) Put the above mixture into a crucible, then burn it in a high-temperature furnace at 1350°C for 4 hours, cool it down to room temperature naturally, take it out, and crush it through a 200-mesh sieve to obtain a yellow long afterglow mate...
Embodiment 3
[0039] A yellow long-lasting luminescent material, using calcium stannate as the luminescent material matrix, using trivalent dysprosium ion Dy 3+ As a luminescent activator, boric acid was used as a flux for material synthesis.
[0040] The above-mentioned preparation method of a yellow long-lasting luminescent material specifically comprises the following steps:
[0041] 1) Calculated as a molar percentage, i.e. CaCO 3 : SnO 2 : Dy 2 o 3 :H 3 BO 3 The ratio of 65.132%: 32.895%: 0.329%: 1.645%, weigh chemically pure CaCO 3 , SnO 2 , Dy 2 o 3 and H 3 BO 3 ;
[0042] 2) Grind the above-mentioned raw materials in a mortar for 1 hour to mix the powder evenly, then pass through a 200-mesh sieve, and press the sieved powder into tablets;
[0043] 3) Put the above mixture into a crucible, then burn it in a high-temperature furnace at 1350°C for 4 hours, cool it down to room temperature naturally, take it out, and crush it through a 200-mesh sieve to obtain a yellow long...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com