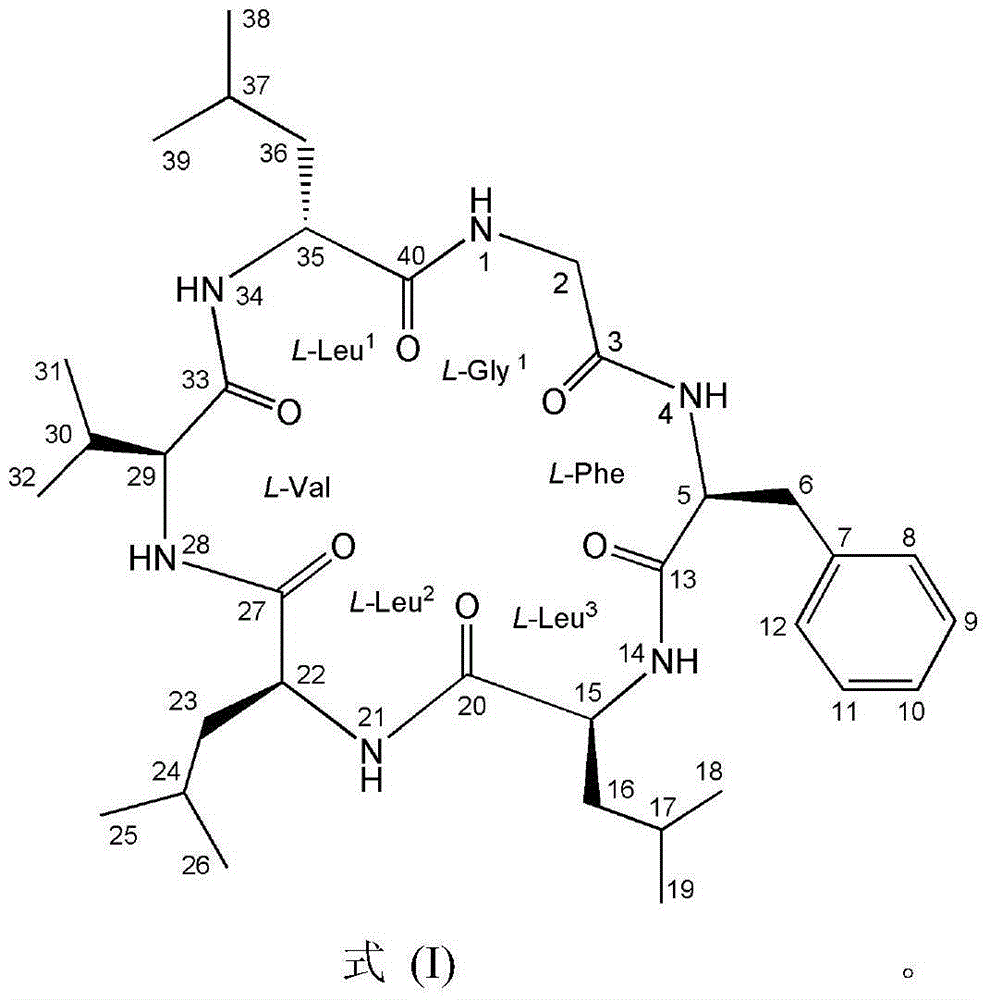
Cyclopeptide compound GG6F and preparation method thereof
A compound and cyclic hexapeptide technology, applied in the field of cyclic hexapeptide compound GG6F and its chemical preparation, can solve problems such as cyclic hexapeptide compounds that have not yet been seen, and achieve the effects of high yield and simple preparation equipment steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 chemically prepares GG6F
[0024] The chemical preparation methods include solid-phase synthesis of linear peptides, liquid-phase ring closure, extraction and column chromatography;
[0025] 1. Synthesis of GG6F linear precursor
[0026] (1) Take the resin: weigh a certain amount of Fmoc-Gly-Wang resin, and swell it in DMF for 15 minutes;
[0027] (2) Remove the Fmoc protecting group: Stir and react with nitrogen in 20% piperidine / DMF solution for 30 minutes; then wash with equal volume of DMF repeatedly for 3 times, each time for 3 minutes;
[0028] (3) Ninhydrin detection: Take a small amount of the reacted resin in a test tube, add 2 drops of 1% (volume fraction, the same below) ninhydrin / ethanol solution, heat for about 2 minutes; the resin is blue-purple or purple-red, It means that the deprotection is successful, proceed to the next step of the reaction; if the resin does not change color, it means that the deprotection is not successful, repeat the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com