Method for synthesizing beta-thiocarbonyl compound by taking Bunte salt as sulfur source
A carbonyl compound and thiocarbonyl technology, applied in the field of synthesizing β-thiocarbonyl compounds, can solve the problems of waste of sulfide, easy to be oxidized, expensive, etc., and achieve the effects of economical and environmental protection, stable operation, and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 1.2mmol S-benzyl (272mg), S-4-methylbenzyl (288mg), S-4-nitrobenzyl (324mg), S-allyl (212mg), S-cyclohexyl ( 348 mg) or one of the S-(2-ethoxy-2-acetyl) Bundt salts (268 mg), 1.0 mmol benzylidene acetone (146 mg) and 0.2 mmol p-toluenesulfonic acid (35 mg) were added in sequence Add 2mL of methanol to the pressure-resistant tube, and stop the reaction after 6h in an oil bath at 80°C. The reaction solvent is removed by rotary evaporation, and the pure target products 1a-1f are obtained by column chromatography on silica gel. The yields are as follows, respectively 90%, 79%, 70%, 73%, 46%, 78%.
[0025]
Embodiment 2
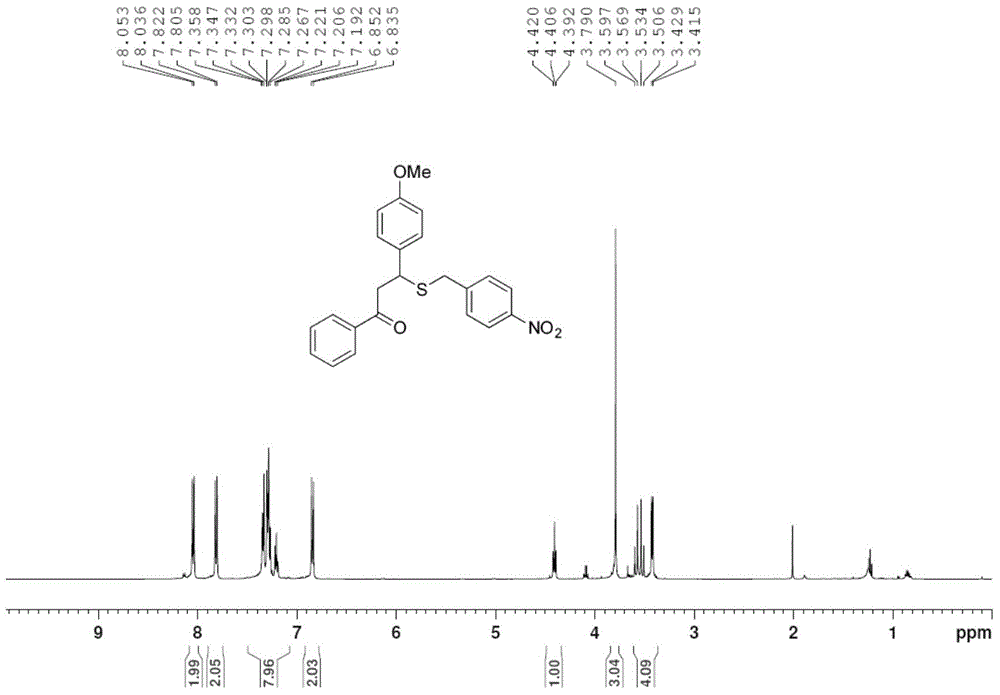
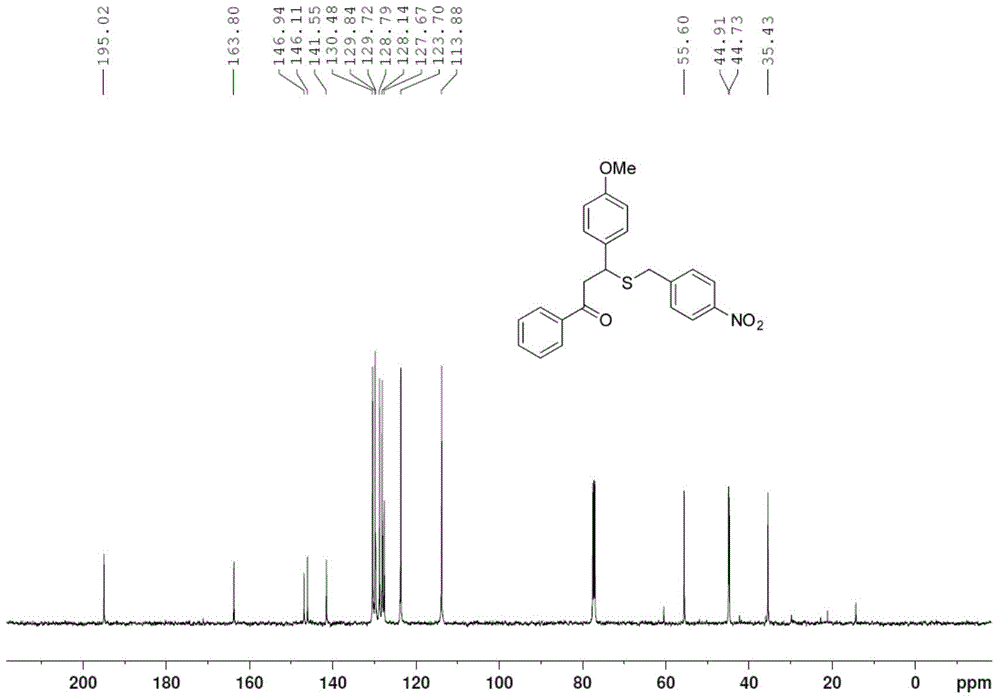
[0027] Take one of 1.0mmol 4'-methylchalcone (222mg), 4'-bromochalcone (286mg) or 4-methoxychalcone (238mg), 1.2mmol of S-4- Add nitrobenzyl bundt salt (324mg) and 0.2mmol p-toluenesulfonic acid (35mg) into the pressure-resistant tube in turn, add 2mL of methanol, and stop the reaction after 6h in an oil bath at 80°C, and remove the reaction solvent by rotary evaporation , through column chromatography on silica gel, the pure target product 1g-1i was obtained, and the yields were 69%, 75%, and 73%, respectively, as shown below. Among them, attached figure 1 , 2 They are the hydrogen and carbon spectra of the new compound 1i, respectively.
[0028]
Embodiment 3
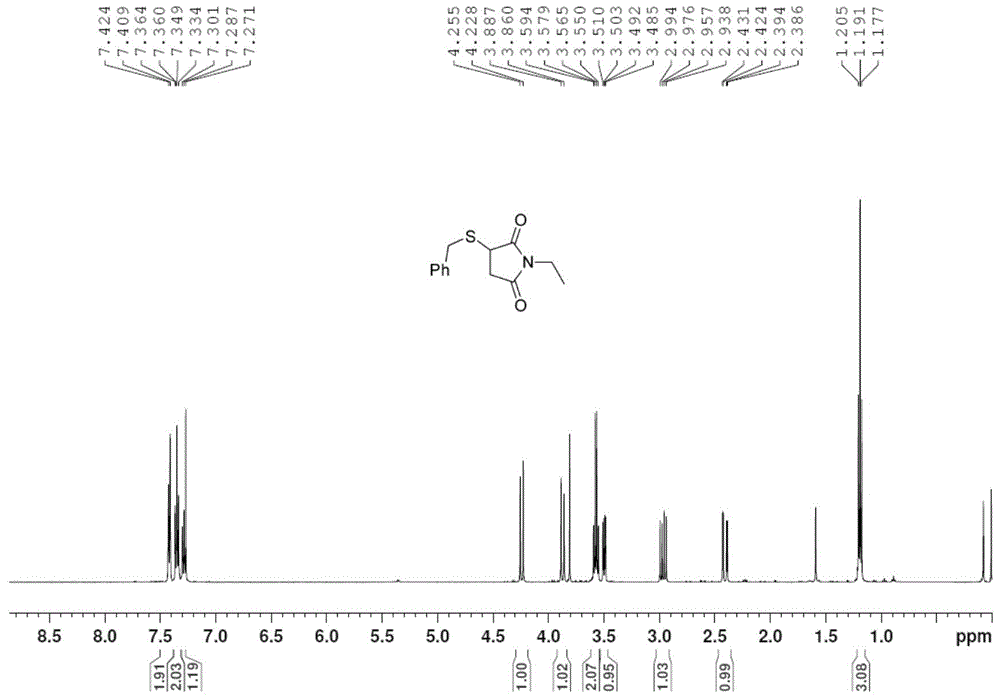
[0030] Take 1.0mmol chalcone (208mg), ethyl acrylate (108μL), 4-(2-thienyl)-3-buten-2-one (152mg), N,N-dimethylacrylamide (100mg ), N-methylmaleimide (112mg), N-ethylmaleimide (126mg) or N-benzylmaleimide (188mg), 1.2mmol of S- Add benzyl bundt salt (272mg) and 0.2mmol p-toluenesulfonic acid (35mg) into the pressure-resistant tube in turn, add 2mL of methanol or dichloromethane, and stop the reaction after 6h in an oil bath at 40°C-80°C. The reaction solvent was removed by rotary evaporation, and the pure target product 1j-1p was obtained by column chromatography on silica gel. The yields were 88%, 93%, 66%, 89%, 76%, 73%, and 95%, respectively, as shown below. Among them, attached image 3 , 4 They are the hydrogen and carbon spectra of the new compound 1o, respectively.
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com