Method for preparing two-dimensional hexagonal boron nitride by using molten state reaction bed
A two-dimensional hexagonal, reactive bed technology is applied in the field of preparation of two-dimensional hexagonal boron nitride, which can solve the problem of damage to the performance of two-dimensional hexagonal boron nitride materials, difficulty in peeling off two-dimensional hexagonal boron nitride, and the size of h-BN single crystal. Small and other problems, to achieve the effect of solving peeling and transfer problems, cost-effective, and easy to manufacture devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
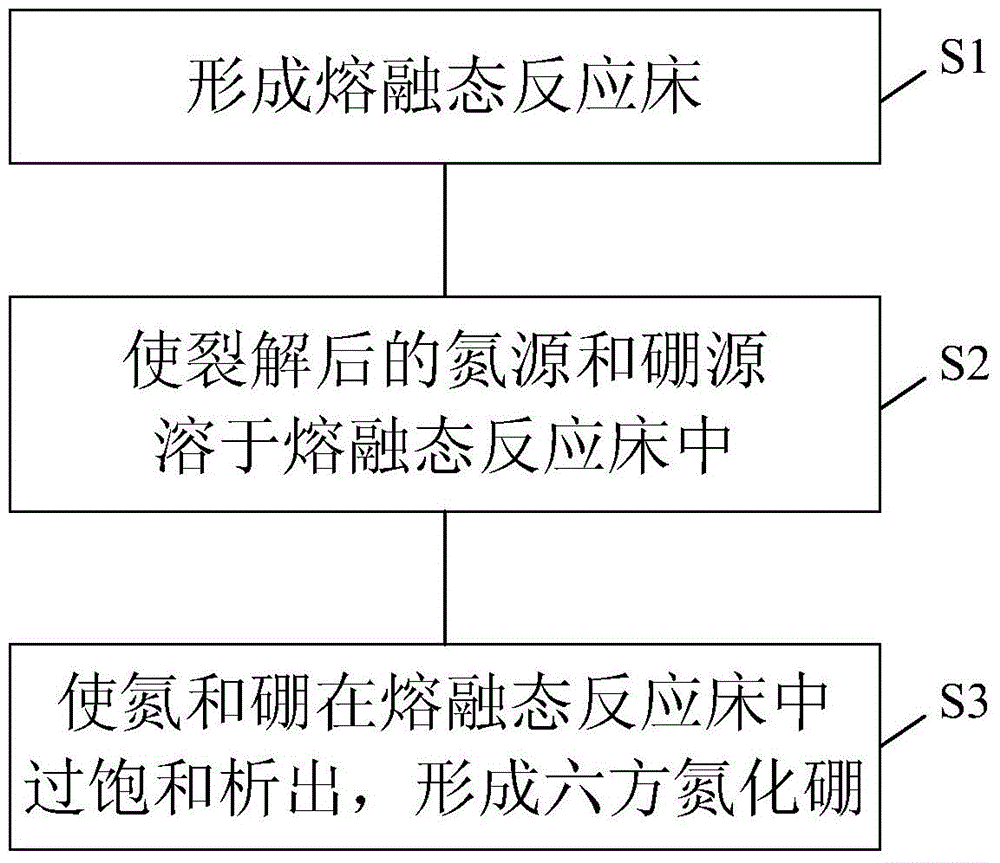
[0036] The substance forming the molten reaction bed is sodium chloride, which is filled into a graphite crucible and placed in a high-temperature vacuum tube furnace. Under normal pressure, feed high-purity ammonia / nitrogen, raise the temperature to 1000°C, then cool down to 900°C and keep it for 5 hours. Mix and pass in continuously. Its reaction equation is as follows:
[0037] BCl 3 +2NH 3 →BN+2HCl+NH 4 Cl
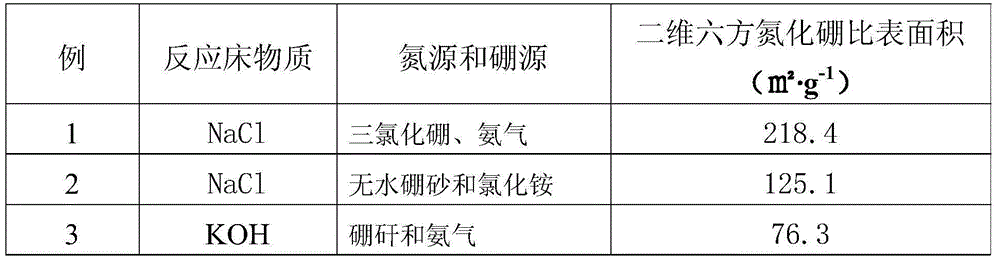
[0038] Then, the temperature of the molten reaction bed is lowered to normal temperature, and the obtained solidified product is eluted with water to obtain a high-purity two-dimensional hexagonal boron nitride material. The specific surface area data of this material are shown in Table 1.
example 2
[0040] The material forming the molten state reaction bed is sodium chloride, dehydrating borax at 450°C and pressure of 79993Pa, drying ammonium chloride at 110-120°C in advance, and then pulverizing them to a fineness of 40 meshes. Sodium chloride, anhydrous borax and ammonium chloride are batched according to the mass ratio of 98.41:1:0.59, mixed and put into a graphite crucible and placed in a high-temperature vacuum tube furnace. Under normal pressure, feed high-purity ammonia / nitrogen, raise the temperature to 1100°C, then cool down to 1050°C and keep it for 5 hours. The reaction equation is as follows:
[0041] Na 2 B 4 o 7 +2NH 4 Cl+2NH 3 →4BN+2NaCl+7H 2 o
[0042] Finally, the temperature of the molten reaction bed was lowered to room temperature. The resulting solidified product is eluted with water to obtain a high-purity two-dimensional hexagonal boron nitride material. The specific surface area data of this material are shown in Table 1.
example 3
[0044] The substance forming the molten state reaction bed is potassium hydroxide, which is dry-mixed with boron gangue and tricalcium phosphate according to the mass ratio of potassium hydroxide, boron gangue and tricalcium phosphate of 92:5:3, and then the mixture is loaded into The graphite crucible is placed in a high temperature vacuum tube furnace. Under normal pressure, feed high-purity ammonia / nitrogen, raise the temperature to 1200°C, then cool down to 1150°C and keep it for 12 hours. The reaction equation is as follows:
[0045] B 2 o 3 +2NH 3 →2BN+3H 2 o
[0046] Finally, the temperature of the molten reaction bed was lowered to room temperature. The resulting solidified product was eluted with water to obtain a high-purity two-dimensional hexagonal boron nitride material, the specific surface area of which is shown in Table 1.
[0047] Table 1
[0048]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com