Amphotericin B nano composite, and preparation method thereof
A nanocomposite and amphotericin technology, applied in the fields of pharmaceutical formulation, drug delivery, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
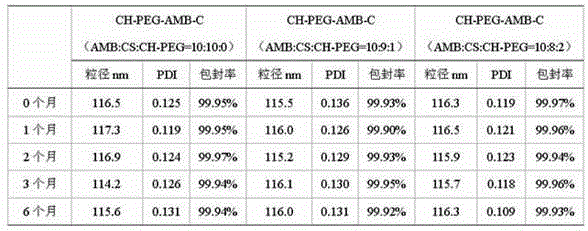
[0023] Preparation of amphotericin B nanocomplexes (organic solvent injection method).
[0024] Accurately weigh amphotericin B, sodium cholesteryl sulfate and cholesterol-PEG with a molar ratio of 10:10:0 or 10:9:1 and dissolve them in an appropriate amount of dimethyl sulfoxide as the oil phase. Prepare 95 g / L lactose, 0.564 g / L tromethamine, and 37.2 mg / L edetate disodium aqueous solution as the water phase. Put the water phase (25°C) in a suitable container, stir it with a mixing emulsifier (1200rpm / min), slowly inject the oil phase into the water phase, control the oil-water ratio to 1:17.5, and continue stirring for 2-3 minutes; The obtained colostrum is ultra-filtered to remove dimethyl sulfoxide; micro-jet is used to sizing to obtain nanocomposites with uniform particle size; and then it is divided into vials and freeze-dried.
Embodiment 2
[0026] Cholesterol-PEG addition range screening.
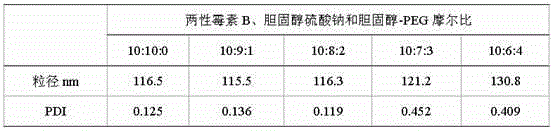
[0027] Adopt the method for Example 1 to prepare amphotericin B, sodium cholesteryl sulfate and cholesterol-PEG molar ratio is 10:10:0, 10:9:1, 10:8:2, 10:7:3, 10:6: 4 amphotericin B nanocomposites, under the same granulation conditions, the particle size was measured after microfluidic sizing, and the results showed that 10:7:3 and 10:6:4 amphotericin B nanocomposites had larger PDI , indicating that the particle size is not uniform, and the particle size of the nano-preparation will affect its safety and effectiveness, so the amount of cholesterol-PEG added in the prescription is limited to less than or equal to 20% (that is, the total molar amount of cholesterol-PEG in cholesterol-PEG and cholesterol sodium sulfate accounted for less than or equal to 20%).
[0028]
[0029]
[0030] Note: PDI is polydispersity index, which reflects the uniformity of particle size.
Embodiment 3
[0032]Accurately weigh amphotericin B, sodium cholesteryl sulfate and mPEG2000-DSPE with a molar ratio of 10:9:1 and dissolve them in an appropriate amount of dimethyl sulfoxide as the oil phase. Prepare 95 g / L lactose, 0.564 g / L tromethamine, and 37.2 mg / L edetate disodium aqueous solution as the water phase. Put the water phase (25°C) in a suitable container, stir it with a mixing emulsifier (1200rpm / min), slowly inject the oil phase into the water phase, control the oil-water ratio to 1:17.5, and continue stirring for 2-3 minutes; The obtained colostrum is ultra-filtered to remove dimethyl sulfoxide; micro-jet is used to sizing to obtain nanocomposites with uniform particle size; and then it is divided into vials and freeze-dried.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com