HPV-integrated gene sites related to occurrence of cervical carcinoma and application thereof
A gene locus, cervical cancer technology, applied in the fields of gynecological oncology and molecular genetics, can solve problems such as poor stability, low sensitivity, and cumbersome technical operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
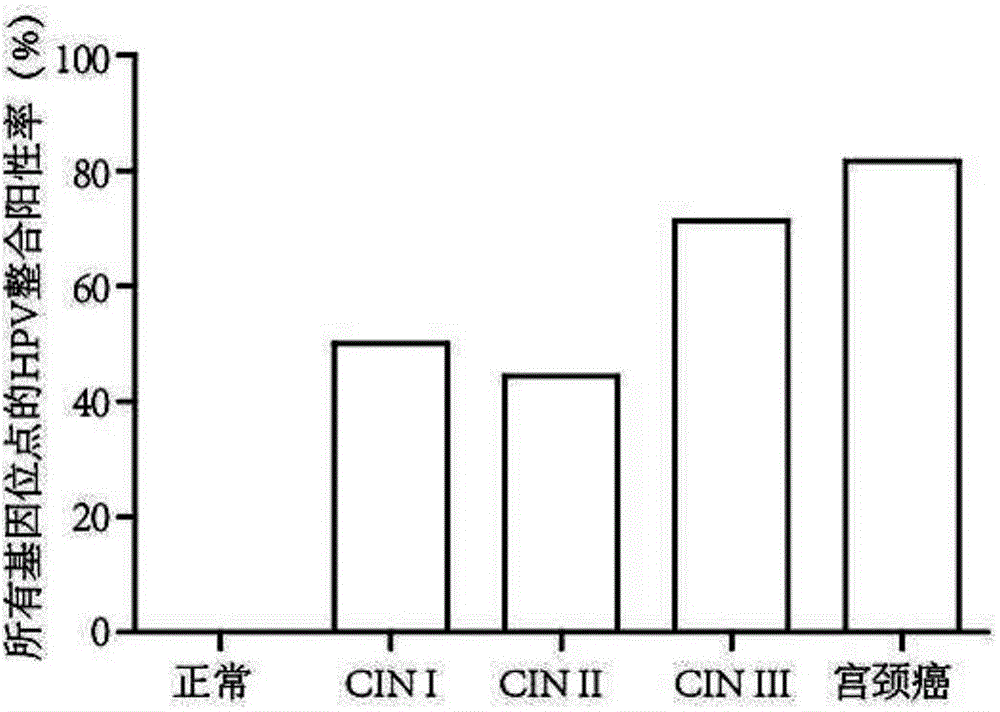
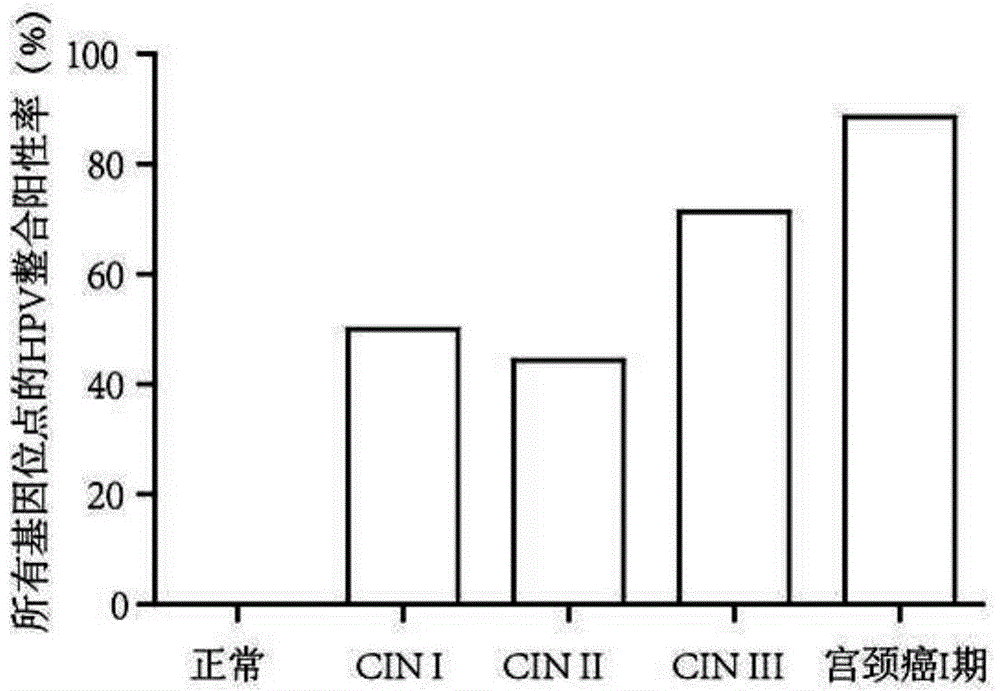
[0103] The cervical tissues of 30 normal people (that is, human cervical tissues not infected by HPV virus), 10 cases of CINI patients, 9 cases of CINII patients, 7 cases of CINIII patients, and 52 cases of cervical cancer were respectively selected. Cervical tissue from stage I patients and 104 cervical cancer patients were used as samples.
[0104] CIN in the present invention refers to cervical intraepithelial neoplasia. CINI, CINII and CINIII refer to different pathological stages of cervical intraepithelial neoplasia.
[0105] 1) Mince 25 mg of cervical tissue, put it in a 1.5 ml tube, add 180 microliters of solution Buffer ATL, shake and mix;
[0106] 2) Add 20 microliters of proteinase K (Proteinase K), shake, and bathe in a 56°C water bath until the cervical tissue is completely dissolved;
[0107] 3) Centrifuge at low speed to shake off the liquid at the top of the tube, add 5 microliters of 100 mg / ml RNAase A, and shake for 15 seconds;
[0108] 4) Add 200 microlit...
Embodiment 2
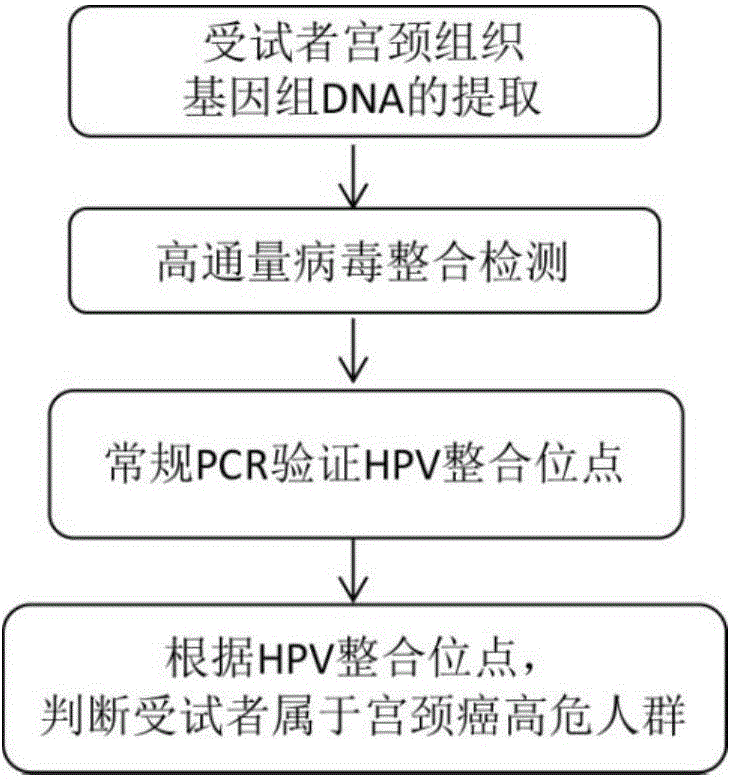
[0115] Detection of HPV integration sites in genomic DNA of cervical tissue using a high-throughput viral integration assay.
[0116] 1) Take the genomic DNA sample of cervical tissue extracted in Example 1 from the -80°C refrigerator, put it in an insulated box with dry ice, and send it to Beijing Maijinuo Technology Gene Technology Co., Ltd. for high-throughput virus integration detection;
[0117] 2) Test the integrity of the sample: prepare an agarose gel with a mass fraction of 1%, take an appropriate amount (about 2 microliters) of the sample and mix it with Syber Green, add it to the agarose gel, and electrophoresis at 150V for 40 minutes in the electrophoresis tank . Exposure in the gel imager shows bands, and a single band without entrainment appears, indicating that the integrity of the sample is relatively good;
[0118] 3) Detection of sample concentration and content: DNA eluent was used as a blank control, and an appropriate amount of sample was taken to detect...
Embodiment 3
[0124] The HPV integration sites were verified by PCR amplification and Sanger sequencing.
[0125] 1. PCR amplification
[0126] Design the upstream and downstream primers that can amplify the HPV integration site, and the primers are as shown in Table 3, prepared according to the following system:
[0127]
[0128] Mix each tube separately, put it in a standard PCR machine and use the following procedure:
[0129]
[0130] 2. Send the amplified PCR product to Wuhan Tian-Huiyuan Biotechnology Co., Ltd. for Sanger sequencing.
[0131] 1) The PCR product obtained after amplification (content greater than 200ng, volume greater than 20ul) was sent to Wuhan Tian-Huiyuan Biotechnology Co., Ltd. for Sanger sequencing;
[0132] 2) The PCR sample is purified, and added to an ABI3730XL sequencer to detect the sequence of the PCR fragment.
[0133] 3. Analyze and detect the integration sites of HPV according to the sequencing results, and detect 50 integration sites.
[0134] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com