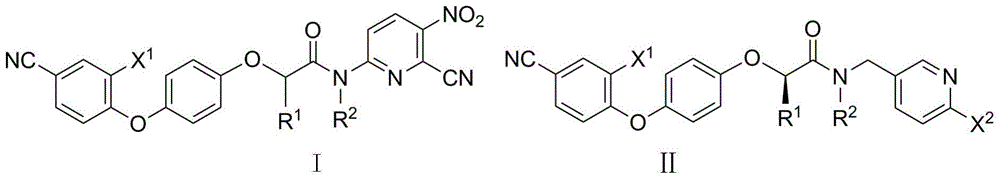
N-pyridyl-2-phenoxy fatty amide and medical application thereof
A technology of phenoxy fatty acid amide and pyridyl, which is applied in the field of compounds, can solve the problems of no research and development reports on the inhibitory activity of influenza virus neuraminidase, and achieve the effect of easy industrial production and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
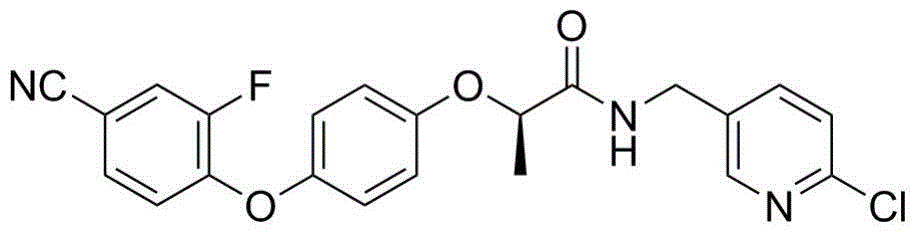
[0021] Preparation of (R)-N-(6-chloropyridine-3-methyl)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionamide
[0022]
[0023] (R)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionyl chloride 3.3mmol, dichloromethane 40mL, 5-aminomethyl-2-chloropyridine 3.3mmol and catalyst Amount of 4-dimethylaminopyridine (DMAP), stirred for 10 min under ice bath, dripped into 10 mmol of triethylamine, continued to stir for 2 h at 10°C, poured into 150 mL of ice water, extracted with dichloromethane, dried, precipitated, and reduced Pressure column chromatography obtains (R)-N-(6-chloropyridine-3-methyl)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propanamide, yield 77.5%, m.p.130.7~131.3℃; 1 H NMR (300MHz, CDCl 3 )δ: 1.60 (d, J=6.9Hz, 3H, CH 3 ), 4.40~4.58 (m, 2H, CH 2 ), 4.74 (q, J=6.9Hz, 1H, CH), 6.87~7.01 (m, 5H, phenyl-H, NH), 7.04 (d, J=6.6Hz, 1H, pyridine ring-H), 7.27 (d, J=6.6Hz, 1H, pyridine ring-H), 7.37~7.56 (m, 3H, phenyl-H), 8.22 (d, J=1.8Hz, 1H, pyridine ring-H); LC-MS , m / z: 4...
Embodiment 2
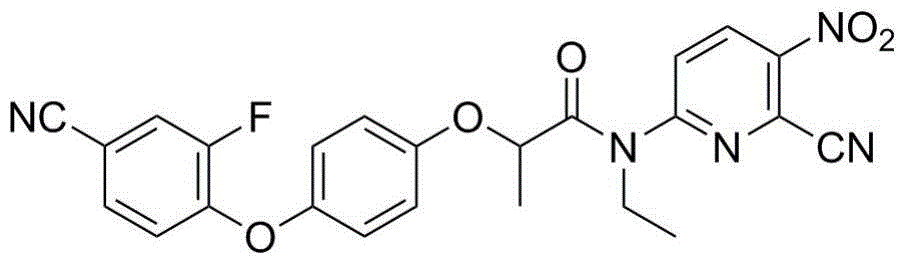
[0025] Preparation of N-ethyl-N-(6-cyano-5-nitropyridin-2-yl)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionamide
[0026]
[0027] 2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionyl chloride (3.3mmol), dichloromethane or 1,2-dichloroethane (40mL), 2-cyano- 6-Amino-3-nitropyridine (3.3mmol) and a catalytic amount of 4-dimethylaminopyridine (DMAP) were stirred for 10min, and triethylamine (1.0g, 10mmol) was added dropwise. Reflux for 8 hours, pour the reaction solution into 150ml of ice water, extract with dichloromethane, dry over anhydrous sodium sulfate, solvent removal, and column chromatography to obtain N-ethyl-N-(6-cyano-5-nitropyridine-2- Base)-2-[4-(4-cyano-2-fluorophenoxy)phenoxy]propionamide, yield 21.2%, m.p.95.7~97.0℃; 1 H NMR (300MHz, CDCl 3 )δ: 1.26(t, J=6.9Hz, 3H, CH 3 ), 1.68 (d, J=6.6Hz, 3H, CH 3 ), 4.06~4.15 (m, 2H, CH 2 ), 5.36 (q, J = 6.6Hz, 1H, CH), 6.74 (d, J = 9.0Hz, 2H, phenyl-H), 6.88 (t, J = 7.8Hz, 1H, phenyl-H), 6.95 (d, J=9.0Hz, 2H, phenyl-H),...
Embodiment 3
[0029] Anti-influenza virus neuraminidase activity of N-pyridyl-2-phenoxy fatty acid amides
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com