Medicine thermal insulation box with precise temperature control function, thermal insulation method and novel application of tetradecane
An incubator and tetradecane technology, applied in chemical instruments and methods, materials for heat exchange, types of packaging items, etc., can solve the problems of drug failure, medical safety, complex operation process, and lower than 0°C, and achieve protection of cold storage The effect of drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
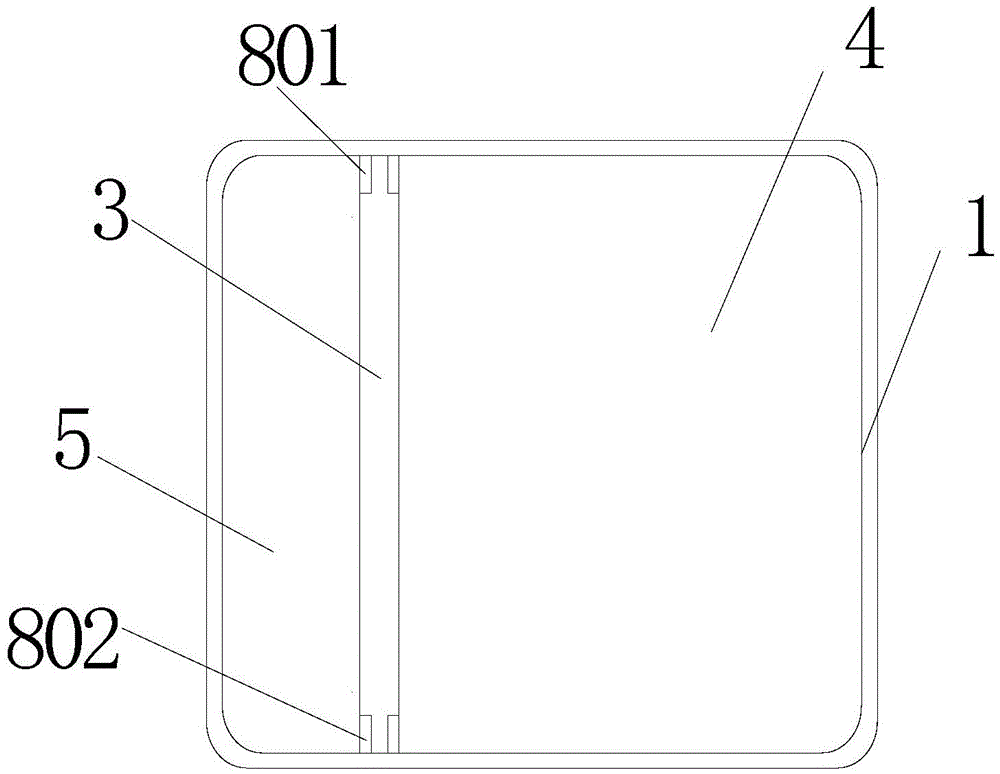
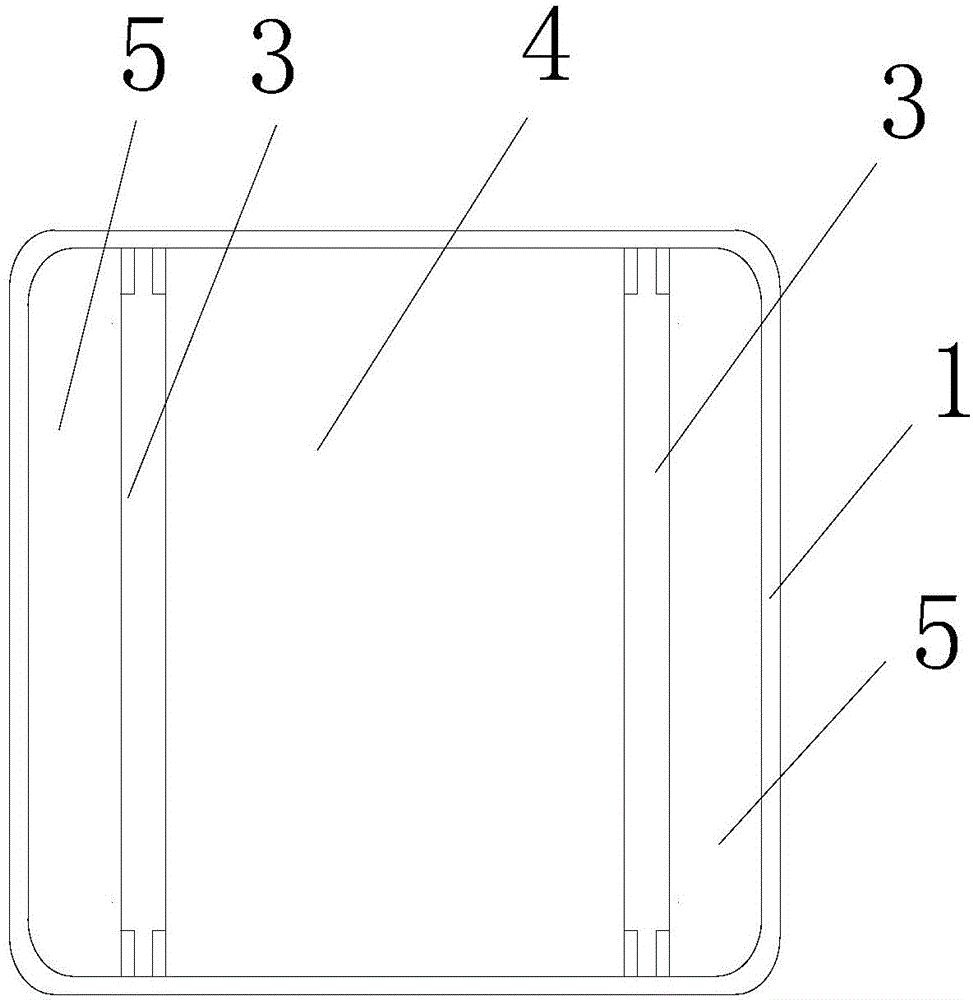
[0040] see figure 1 and figure 2 , the precise temperature control drug incubator of the present embodiment includes an incubator box 1 provided with a cavity and an incubator upper cover 2 that can be covered on the incubator box;
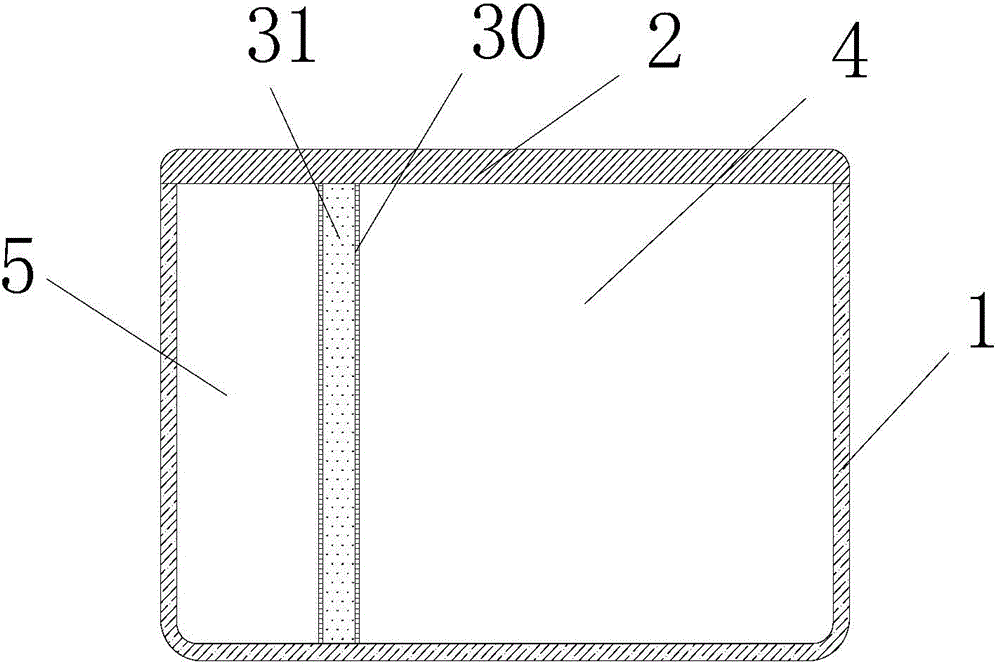
[0041] The accommodating cavity is divided into a medicine accommodating cavity 4 and a cold source accommodating cavity 5 by a heat storage partition 3;
[0042] The heat storage separator 3 includes a casing 30 and a heat storage agent 31 sealed in the casing 30 with a phase transition temperature of 3-7°C.
[0043] The precise temperature-controlled medicine incubator of the present embodiment or the medicine antifreeze and heat preservation method of the present invention, when used in the hot season when the daily average temperature of the environment is higher than 8 degrees Celsius, put the frozen phase transition temperature in the cold source accommodation chamber 5. A low-temperature cold source at about 0°C is placed in the medicine...
Embodiment 2
[0051] The present embodiment provides a kind of antifreeze and heat preservation method for medicines in the hot season when the average daily temperature of the environment is higher than 8 degrees Celsius, comprising the following steps:
[0052] S10, sealing a heat storage agent with a phase change temperature of 3-7°C in the casing to form a heat storage separator;
[0053] S20, placing the heat storage separator in a normal temperature environment with a temperature higher than 8°C, so that the heat storage agent therein absorbs the heat of the environment and presents a liquid state;
[0054] S30. Place the heat storage partition in the cavity of the incubator, and divide the cavity inside the incubator into a cold source cavity and a medicine cavity;
[0055] S40. Put a frozen low-temperature cold source with a phase transition temperature of about 0°C in the cold source storage cavity, and put refrigerated medicines at 0-10°C taken out of the refrigerated storage devi...
Embodiment 3
[0059] This embodiment provides a method for antifreezing and heat preservation of refrigerated medicines in the cold season when the daily average temperature of the environment is lower than 8 degrees Celsius, comprising the following steps:
[0060] D10. Seal the heat storage agent with a phase change temperature of 3-7°C in the shell to make a heat storage separator;
[0061] D20. Place the heat storage separator in an environment with a temperature higher than 8°C, so that the heat storage agent in it absorbs the heat of the environment and presents a liquid state;
[0062] D30. Place the heat storage partition in the cavity of the incubator, and divide the cavity inside the incubator into a cold source cavity and a refrigerated medicine cavity;
[0063] D40. Put a low-temperature cold source with a phase transition temperature of about 0°C that has been stored in an environment with a temperature higher than 8°C in the cold source storage cavity; Refrigerated medicines ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com