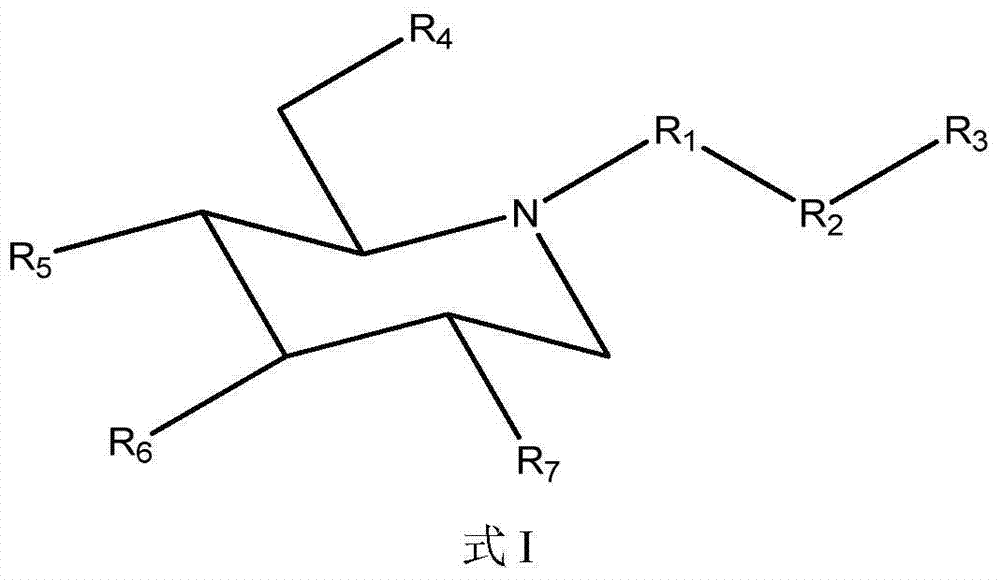
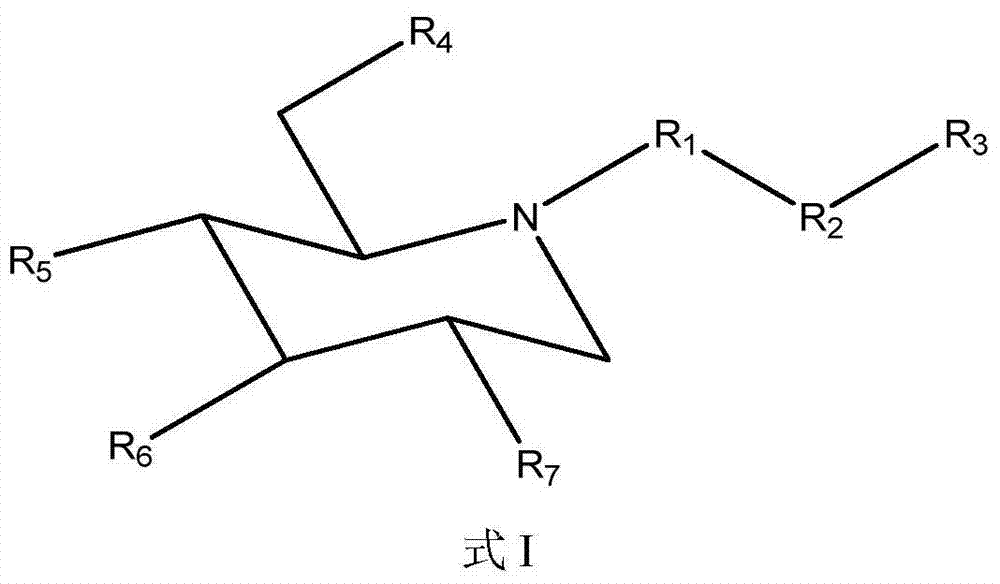
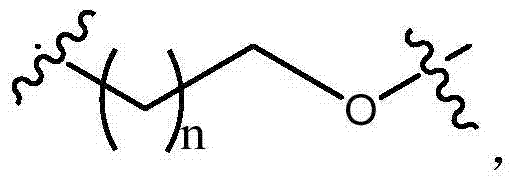Compositions and methods for treatment of hyperglycemia
A composition and compound technology, applied in the direction of drug combination, active ingredients of heterocyclic compounds, blood diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0154] The comparison of the compound of formula I (1-3) and eicosapentaenoic acid (EPA) solubility in water
[0155]Measurement of aqueous solubility of test compounds is accomplished by methods well known to those skilled in the art. Specifically, distilled water was added several times in small amounts to a weighed amount of the test compound of the example compound formula I (1-3) until a clear solution was obtained. Measure the total volume of the solution. Water solubility was calculated by dividing the weight of the salt in milligrams (mg) by the volume of the solution in mL. When measured by the above method, the water solubility of the compound of formula I (1-3) was determined to be 68.6 mg / ml. Likewise, the water solubility of EPA was found to be <0.2 mg / mL. Thus, compounds of formula I (1-3) are at least 260 times more water soluble than EPA itself. This clearly demonstrates the unexpectedly high bioavailability of the composition of the invention. When a high...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com