Phenothiazine derivatives and their use in treating pulmonary tuberculosis
A technology of derivatives and uses, applied in medical preparations containing active ingredients, pharmaceutical formulas, organic chemistry, etc., can solve problems such as limiting antibacterial efficacy and adverse central nervous system side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0037]
[0038] In a more preferred embodiment, the tricyclic derivative of general formula (1) can be selected from:
[0039]
[0040] The process of preparing N-alkyl sulfonate of phenothiazine includes:
[0041] (a) Preparation of phenothiazine anions, and
[0042] (b) The reaction of the anion with a cycloalkyl sulfonate (e.g. 1,3-propane sultone or 1,4-butane sultone) is roughly described in US Patent 7,855,287.
[0043] An inefficient multi-step synthesis method was earlier disclosed in Journal of Physical Chemistry, 1986, 90, 2469-2415.
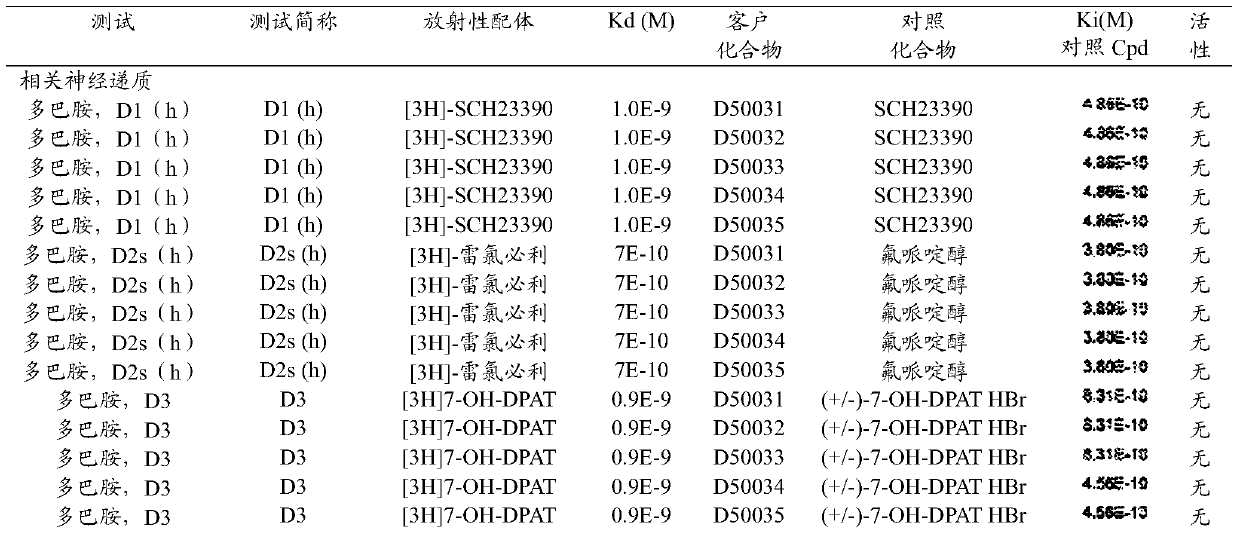
[0044] The phenothiazine derivatives of the present invention have been shown to have limited toxicity to primary macrophage cell cultures and limited psychoactive activity.
[0045] It is believed that the anti-schizophrenic activity of phenothiazine drugs is related to the blockage of synaptic dopamine receptors in the brain. It has been shown by molecular space-filling models that the favorable van der Waals interaction between the side chain...
Embodiment
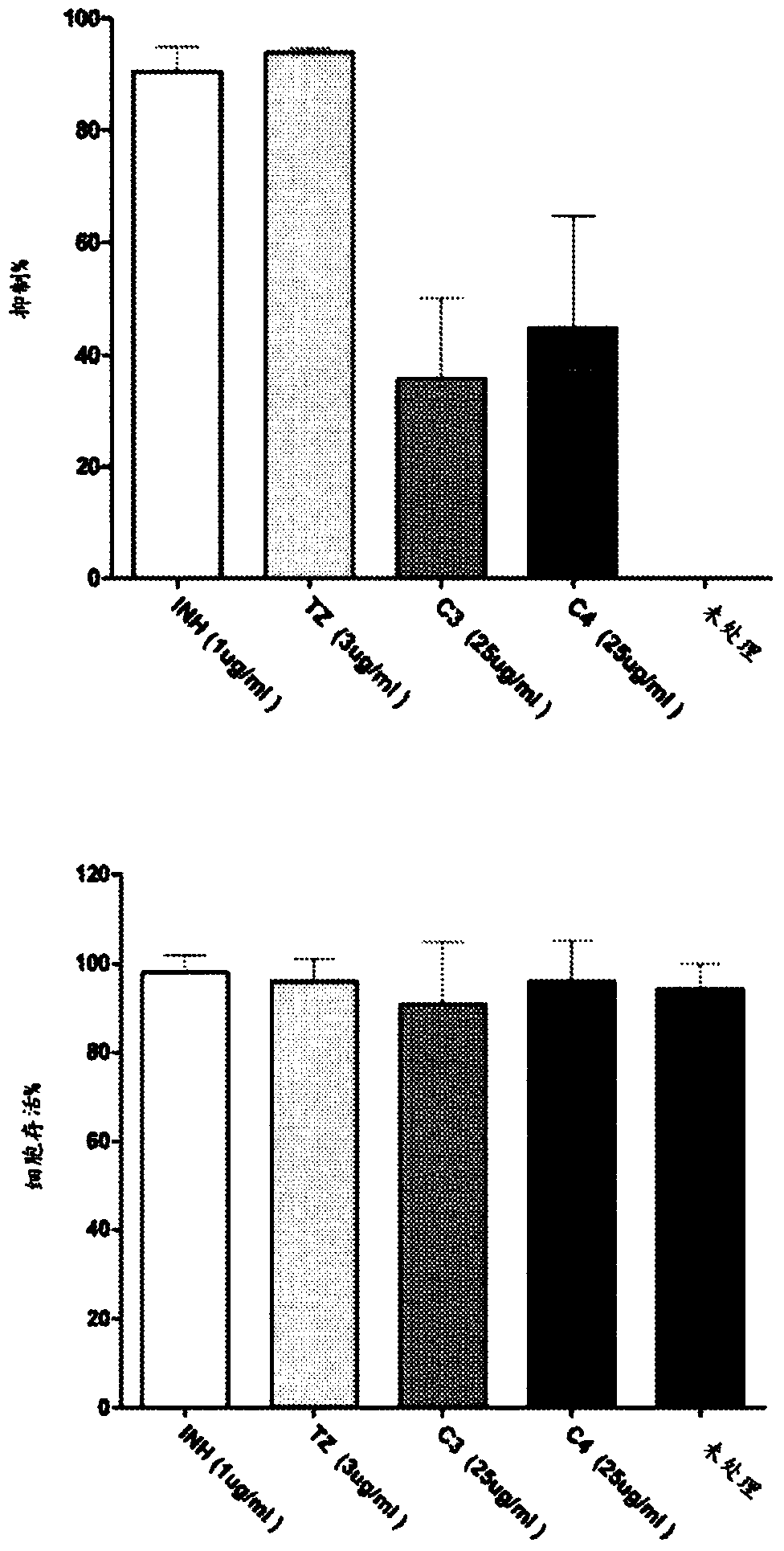
[0057] Phenothiazine derivatives show direct antibacterial activity against Mycobacterium tuberculosis in culture
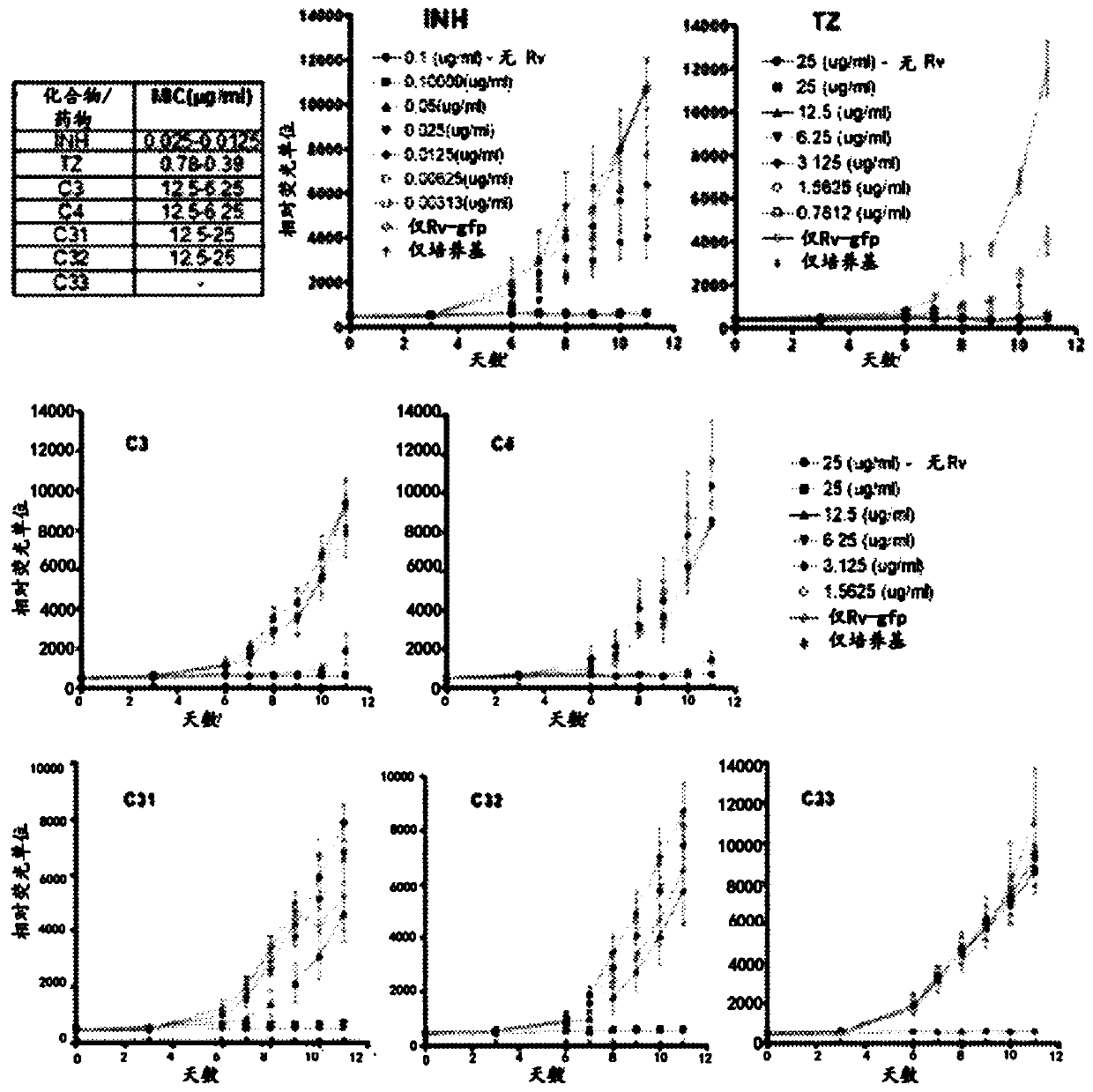
[0058] The GFAP microplate assay (GFPMA) was used to evaluate the five compounds C3, C4, C31, C32 and C33 (shown below) against Mycobacterium tuberculosis in order to screen for the bactericidal / bacteriostatic activity of phenothiazine derivatives.
[0059]
[0060] The results were compared with the known phenothiazine derivative thioridazine (TZ) and the first line drug isoniazid (INH), which was used as a positive control for anti-mycobacterial efficacy. The results obtained have shown that, except for C33, all test compounds showed a dose-dependent inhibitory effect on the growth of M.tb H37Rv.gfp, and the obtained MICs were summarized 50 value( figure 1 ). MIC of INH and TZ 50 The value is similar to the published data. The significant anti-tuberculosis activity of C3 and C4 was confirmed, which has the lowest MIC, as did C31 and C32.
[0061] Phenothiazine der...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com