Oxidation reduction shuttle used as lithium ion battery overcharge protecting agent
A lithium-ion battery and electrolyte additive technology, applied in the field of electrochemical energy storage, can solve the problems of reducing molecular stability, unable to provide overcharge protection, etc., and achieve the effect of improving oxidation potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1. The preparation of the redox shuttle used as an overcharge protection agent for lithium-ion batteries
preparation Embodiment 1
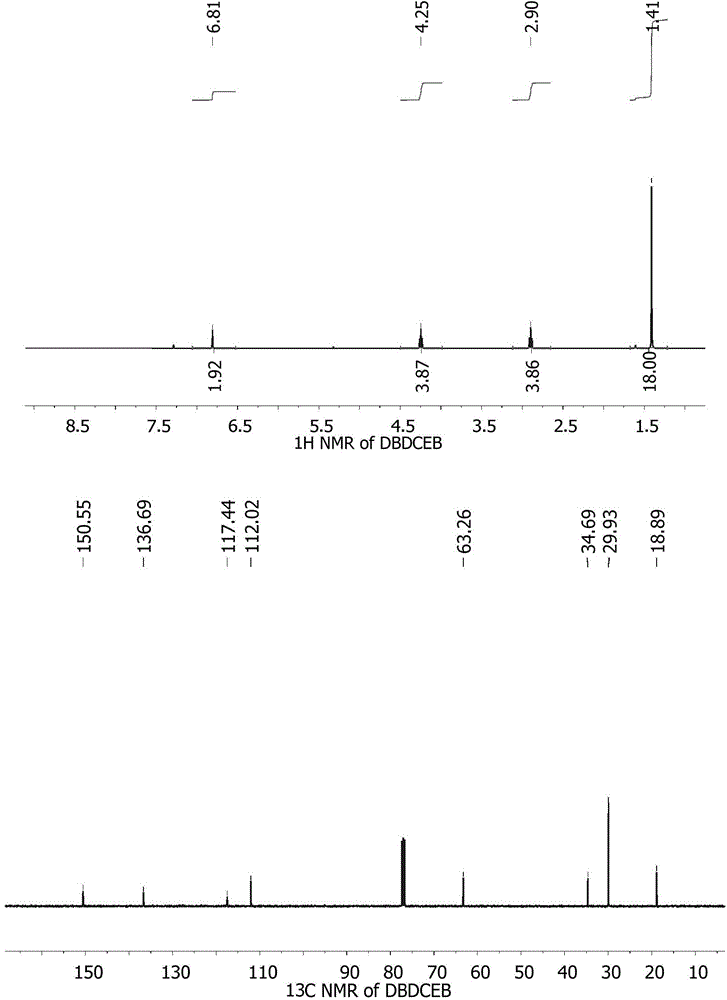
[0022] Preparation Example 1: Synthesis of 2,5-di-tert-butyl-1,4-dinitrile propoxybenzene (DBDCEB)
[0023]
[0024] Add 2,5-di-tert-butylhydroquinone (0.1mol, 222.32g / mol, 22.23g), acrylonitrile (1mol, 53.06g / mol, 53.06g), 5% Potassium carbonate solution (0.01mol, 138.21g / mol, 1.4g) and 10% isobutanol (0.02mol, 74.12g / mol, 1.48g). The resulting mixture was heated to reflux for 8 hours. Another 5% potassium carbonate solution (0.01mol, 138.21g / mol, 1.40g) was added to the reaction system, and after 36 hours of reaction, 85% phosphoric acid (0.016mol, 98.0g / mol, 1.84 g) Stir for half an hour to neutralize. Excess acrylonitrile was removed by distillation under reduced pressure. The obtained crude product was diluted with dichloromethane (75 mL) and washed with 0.1M NaOH solution. After the organic layer was concentrated, the pure product was obtained by recrystallization, yield: 70%.
[0025] 1 H NMR (400MHz, CDCl 3 ): δ / ppm, 6.81(s, 2H, Ar-H), 4.25(s, 4H, CH 2 CN),2...
preparation Embodiment 2
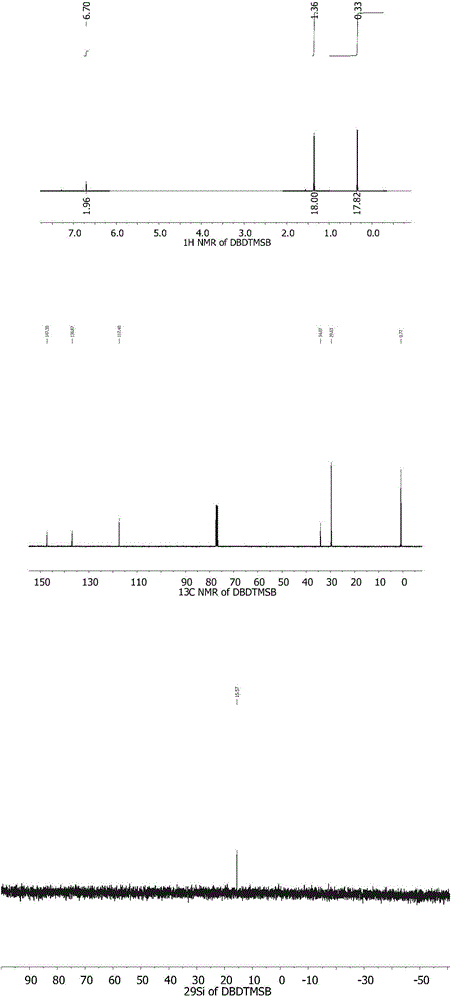
[0027] Preparation Example 2: Synthesis of 2-5-di-tert-butyl-1,4-ditrimethylsilyloxybenzene (DBDTMSB)
[0028]
[0029] Add 2,5-di-tert-butylhydrobenzoquinone (0.1mol, 222.32g / mol, 22.23g) and acetonitrile (100mL) into a flame-dried three-neck flask. After hexamethyldisilazane (HMDS) was added dropwise to the reaction system, it was heated to reflux for 16 hours. The pure product was obtained by recrystallization, yield: 85%.
[0030] 1 H NMR (400MHz, CDCl 3 ): δ / ppm, 6.70(s, 2H, Ar-H), 1.36(s, 18H, -C(CH 3 ) 3 ),0.33(s,18H,-Si(CH 3 ) 3 );
[0031] 13 C NMR (100MHz, CDCl 3 ):147.30,136.87,117.40,34.07,29.63,0.77;
[0032] 29 Si NMR (50MHz, CDCl 3 ): 15.56.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com