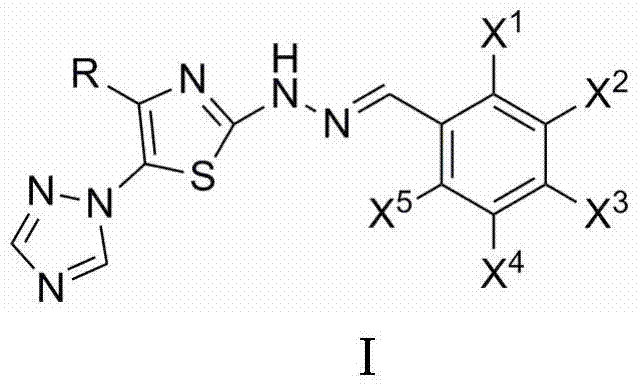
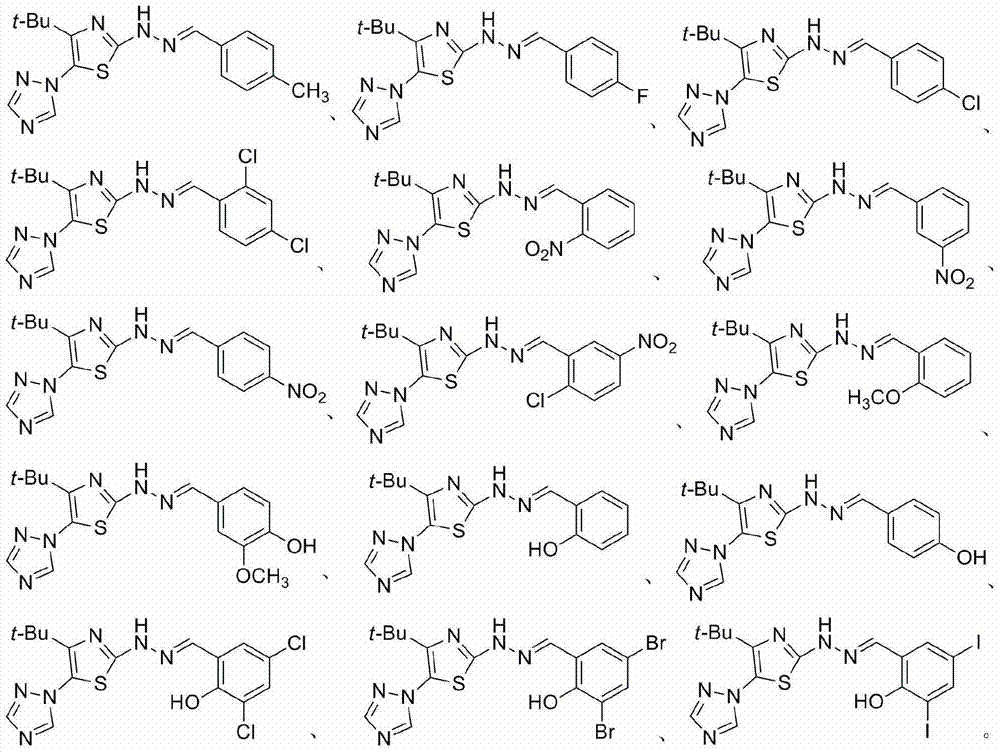
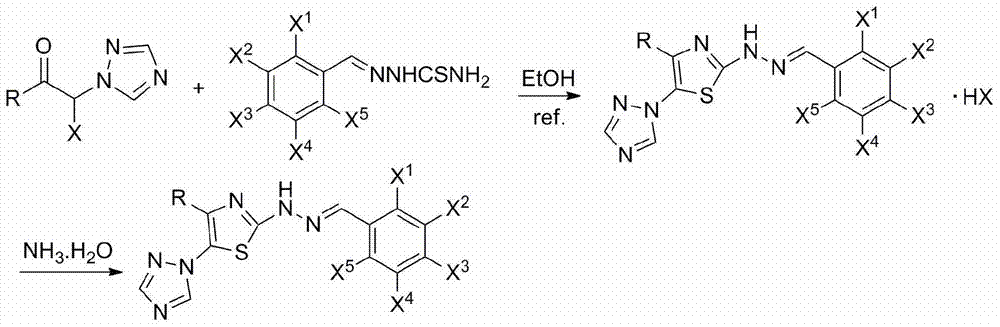
2-(2-benzylidene hydrazino)-5-(1,2,4-triazole-1-yl)thiazole, and preparation and applications thereof
A kind of technology of nitrobenzyl hydrazide group and hydroxybenzyl hydrazide group, which is applied in the application field of preparing influenza virus neuraminidase inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-1-bromo-2-butanone
[0020]0.02mol3,3-dimethyl-1-(1,2,4-triazol-1-yl)-2-butanone, 4.0mL glacial acetic acid, 40.0mL carbon tetrachloride, 0.022mol sodium bromide, 6.0 Add 1.2 mL of sulfuric acid, add 4.6 mL of 30% hydrogen peroxide dropwise at 65°C, and react for 2.5 hours; add 20.0 mL of aqueous sodium bicarbonate solution, stir until no bubbles emerge, separate the organic layer, extract with dichloromethane, and combine The organic layer was washed with saturated sodium chloride and dried over anhydrous sodium sulfate. The solvent was removed to obtain a light yellow liquid of 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-1-bromo-2-butanone; the light yellow liquid was directly used in the next reaction.
Embodiment 2
[0022] Preparation of 2-[2-(4-methylbenzylhydrazono)]-4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazole and its hydrobromide
[0023] 5mmol thiosemicarbazide was dissolved in 20.0mL mixed solvent (V 乙醇 :V 水 =1:3), reflux, add 5mmol of 4-methylbenzaldehyde ethanol solution dropwise, react for 0.5h, the reaction solution is cooled to precipitate solid, filtered, dried, ethanol recrystallization to obtain 4-methylbenzylhydrazonothioamide , yield 82.5%, m.p.165~167℃.
[0024] 5mmol of 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-1-bromo-2-butanone was dissolved in 20.0mL of ethanol, and 5mmol of 4-methylbenzylhydrazonosulfur was added Substituted amide, reflux, reacted for 0.5h, the reaction solution was cooled to precipitate a solid, filtered to obtain 2-[2-(4-methylbenzylhydrazono)]-4-tert-butyl-5-(1,2,4- Triazol-1-yl)thiazole hydrobromide, yield 76.6%, m.p.221~223℃. 1 H NMR (400MHz, CDCl 3 ) δ: 1.29 (s, 9H, 3 × CH 3 ), 2.41 (s, 3H, CH 3 ), 7.25 (d, J=8.0Hz, 2H, C 6 h 4 3,5-H), 7.57 (...
Embodiment 3
[0027] Preparation of 2-[2-(4-fluorobenzylhydrazono)]-4-tert-butyl-5-(1,2,4-triazol-1-yl)thiazole and its hydrobromide
[0028] 5mmol thiosemicarbazide was dissolved in 20.0mL mixed solvent (V 乙醇 :V 水 =1:3), reflux, add 5mmol of 4-fluorobenzaldehyde ethanol solution dropwise, react for 1.0h, the reaction liquid is cooled to precipitate solid, filtered, dried, ethanol recrystallization to obtain 4-fluorobenzylhydrazonothioamide, yield The rate is 93.1%, m.p.195~198℃.
[0029] Dissolve 5mmol of 3,3-dimethyl-1-(1,2,4-triazol-1-yl)-1-bromo-2-butanone in 20.0mL of ethanol, add 5mmol of 4-fluorobenzylhydrazonothio Amide, reflux, react for 0.2h, the reaction solution is cooled to precipitate a solid, filtered to obtain 2-[2-(4-fluorobenzylhydrazono)]-4-tert-butyl-5-(1,2,4-triazole -1-yl) thiazole hydrobromide, yield 86.8%, m.p.208~211℃. 1 H NMR (400MHz, CDCl 3 ) δ: 1.30 (s, 9H, 3×CH 3 ), 7.14 (t, J=8.8Hz, 2H, C 6 h 4 3,5-H), 7.69 (dd, J=8.8Hz, J=1.6Hz, 2H, C 6 h 4 2,6-H), 8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com