A kind of analytical detection method of valganciclovir hydrochloride impurity
A valganciclovir hydrochloride and detection method technology, which is applied in the field of analysis and detection of valganciclovir hydrochloride impurities, can solve problems such as separation difficulties, and achieve the effects of high sensitivity, good linear relationship, and good accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
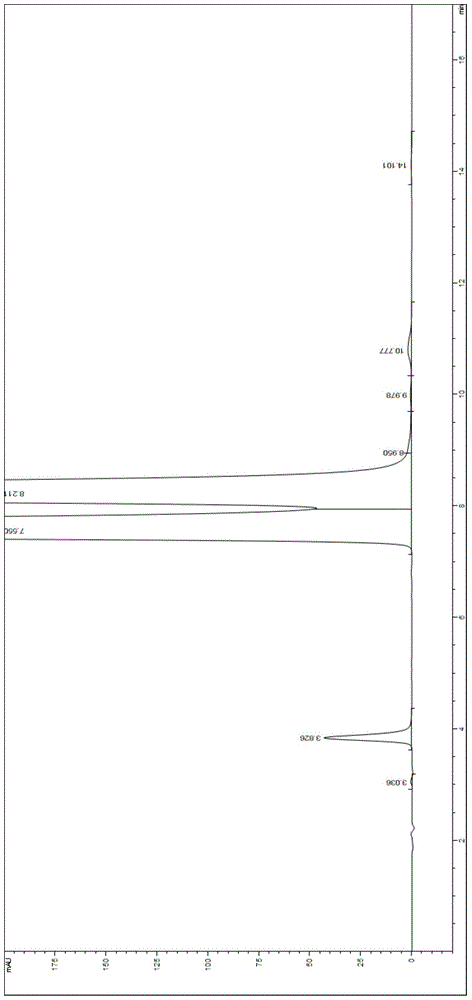
Embodiment 1
[0042] Chromatographic conditions: use phenylsilane-bonded silica gel as the filler, and the mobile phase is triethylamine ammonium formate buffer with pH=5.6 (in addition to water, it contains three components, triethylamine, ammonium formate and trifluoroacetic acid, and its content Respectively: 0.2% (v / v) triethylamine, 0.05mol / L ammonium formate and an appropriate amount of trifluoroacetic acid, wherein an appropriate amount of trifluoroacetic acid is used to adjust the pH of the buffer to 5.6)-methanol, the The volume ratio of buffer to methanol was 95:5, the flow rate was 1.0 ml / min, the detection wavelength was 254 nm, the chromatographic column: COSMOSIL 5PE-MS 150×4.6 mm, 5 μm, the column temperature was 35 °C, and the injection volume: 50 μl.
[0043] Preparation of the test solution:
[0044] Take about 50 mg of valganciclovir hydrochloride crude drug, accurately weigh it, put it in a 100 ml volumetric flask, add 0.001 mol / L hydrochloric acid to dissolve and dilute...
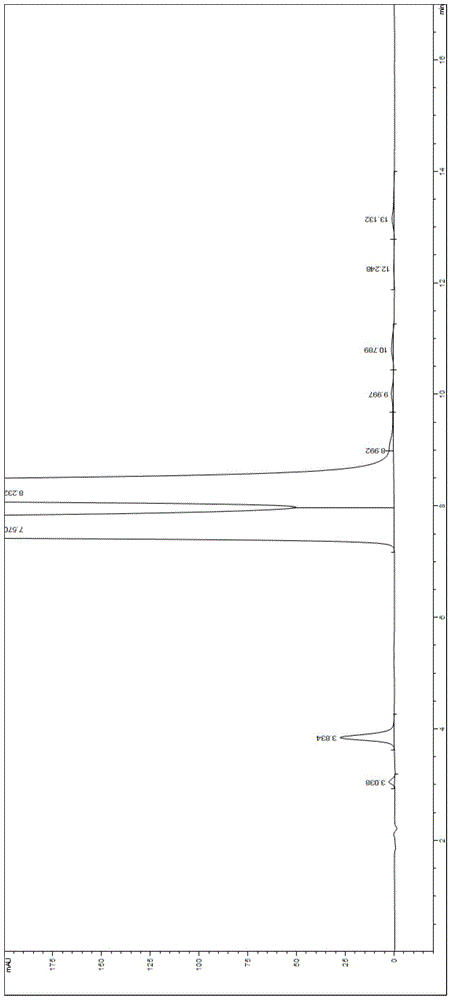
Embodiment 2
[0052] Chromatographic conditions: use phenylsilane-bonded silica gel as filler, and the mobile phase is triethylamine ammonium acetate buffer with pH=5.3 (in addition to water, it contains three components, triethylamine, ammonium acetate and formic acid, whose contents are : 0.25% (v / v) triethylamine, 0.03 mol / L ammonium acetate and an appropriate amount of formic acid, wherein an appropriate amount of formic acid is used to adjust the pH value of the buffer to 5.3)-methanol, the volume of the buffer and methanol The ratio was 95:5, the flow rate was 1.0 ml / min, the detection wavelength was 254 nm, the chromatographic column: COSMOSIL5PE-MS 150×4.6 mm, 5 μm, the column temperature was 35° C., and the injection volume was 50 μl.
[0053] Preparation of the test solution:
[0054] Take about 50mg of valganciclovir hydrochloride raw material, accurately weigh it, put it in a 100ml volumetric flask, add an appropriate amount of each of the six impurities, and prepare it into gua...
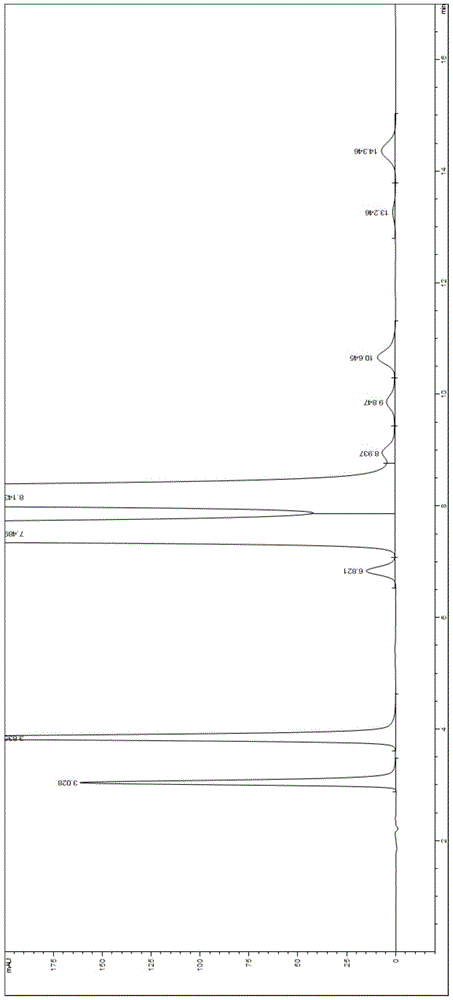
Embodiment 3
[0059] Chromatographic conditions: use phenylsilane-bonded silica gel as filler, and the mobile phase is triethylamine ammonium acetate buffer with pH=5.4 (in addition to water, it contains three components, triethylamine, ammonium acetate and trifluoroacetic acid. Respectively: 0.35% (v / v) triethylamine, 0.03 mol / L ammonium acetate and an appropriate amount of trifluoroacetic acid, wherein an appropriate amount of trifluoroacetic acid is used to adjust the pH of the buffer to 5.4)-methanol, the The volume ratio of buffer to methanol was 96:4, the flow rate was 1.0 ml / min, the detection wavelength was 254 nm, the chromatographic column: COSMOSIL 5PE-MS 150×4.6 mm, 5 μm, the column temperature was 30 °C, and the injection volume was 50 μl.
[0060] Preparation of the test solution:
[0061] Take valganciclovir hydrochloride tablets, grind them finely, accurately weigh an appropriate amount (approximately equivalent to 50 mg of the main drug containing valganciclovir hydrochlori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com