Method for synthesizing 3,5-diphenyl phenol
A diphenylphenol and reactor technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problem of limiting the synthesis of ortho-substituted derivatives, easy formation of isomers, and limited selection of raw materials and other problems, to achieve the effect of improving the total yield of the reaction, optimizing the reaction, and easily expanding the substrate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
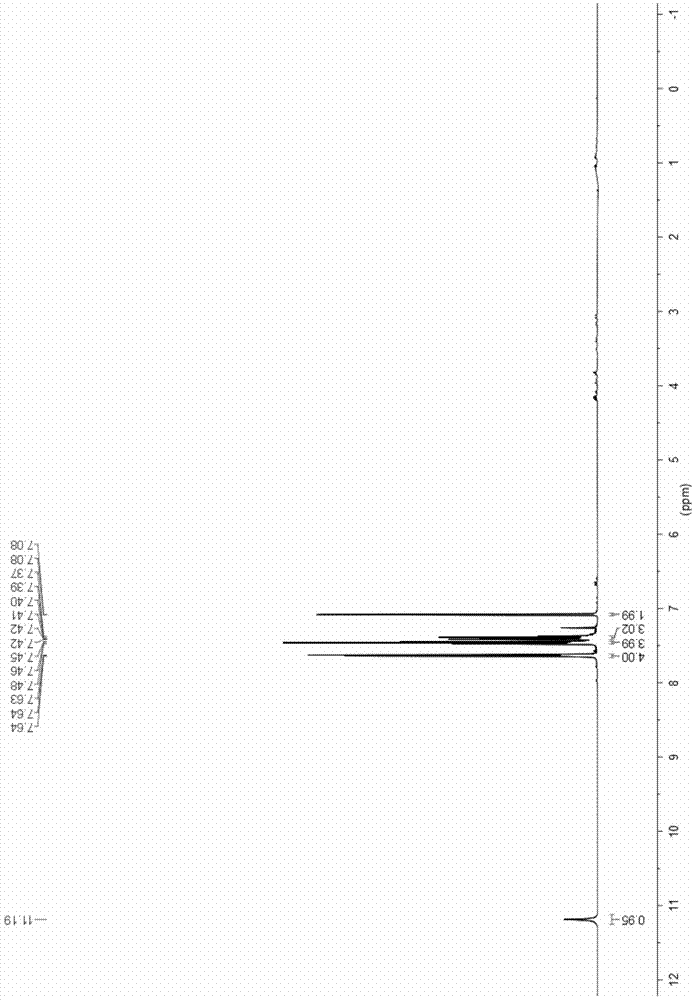
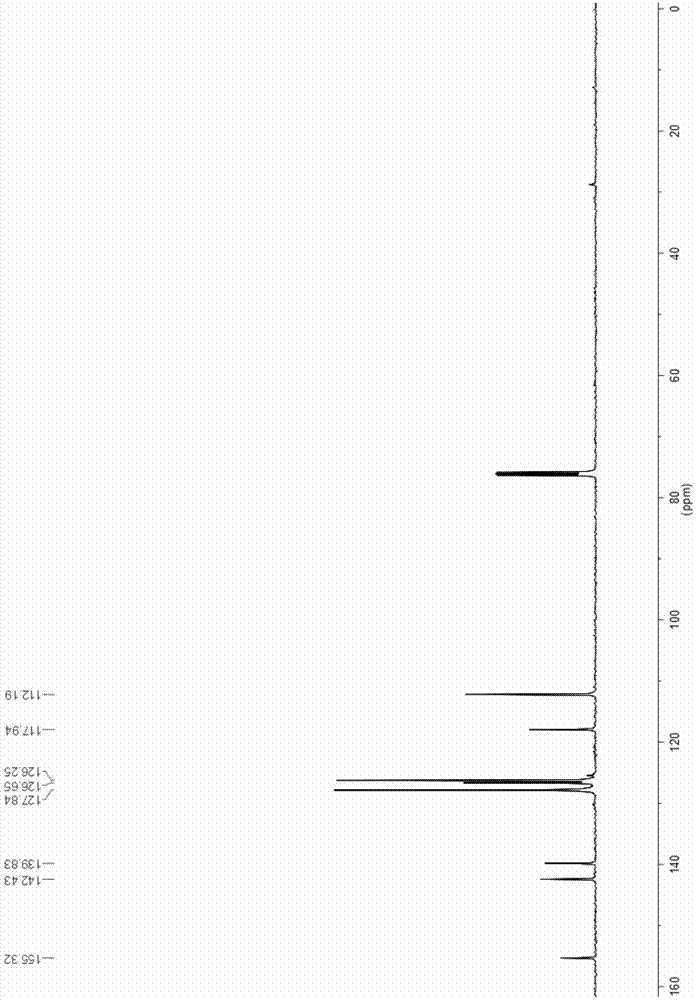
[0032] Add 130mg (1mmol) ethyl acetoacetate, 390mg (1.1mmol) Selectfluor TM , 2mL of acetonitrile, reflux at 85°C for 2h to obtain a yellow mixed solution; continue to add 208mg (1mmol) of chalcone and 652mg (2mmol) of cesium carbonate to the pressure tube, and react at 120°C for 2h to obtain a brownish yellow reaction solution. Add 2M dilute hydrochloric acid dropwise to adjust the pH of the reaction solution to 5-6, and the color of the reaction solution becomes lighter. Solvent acetonitrile was removed by distillation under reduced pressure, and the yellow organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, and dichloromethane was removed by distillation under reduced pressure to obtain a crude product, which was subjected to column chromatography (ethyl acetate:petroleum ether). =1:4) separated and purified to obtain 204.5 mg of white solid, with a total yield of 83%. For its structural characterization, see figure 1 and figure 2 .
...
Embodiment 2
[0035] Add 130mg (1mmol) ethyl acetoacetate, 390mg (1.1mmol) Selectfluor TM , 2mL of acetonitrile, reacted at 80°C for 3h to obtain a yellow mixed solution; continued to add 208mg (1mmol) of chalcone and 652mg (2mmol) of cesium carbonate to the pressure tube, and reacted at 110°C for 4h to obtain a brownish yellow reaction solution. Add 2M dilute hydrochloric acid dropwise to adjust the pH of the reaction solution to 5-6, and the color of the reaction solution becomes lighter. Solvent acetonitrile was removed by distillation under reduced pressure, and the yellow organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, and dichloromethane was removed by distillation under reduced pressure to obtain a crude product, which was subjected to column chromatography (ethyl acetate:petroleum ether). =1:6) separated and purified to obtain 197.1 mg of white solid with a total yield of 80%.
Embodiment 3
[0037] Add 130mg (1mmol) ethyl acetoacetate, 390mg (1.1mmol) Selectfluor TM, 2 mL of acetonitrile, reacted at 90°C for 2 hours, and obtained a yellow mixed liquid; continued to add 208 mg (1 mmol) of chalcone and 652 mg (2 mmol) of cesium carbonate to the pressure tube, and reacted at 130° C. for 2 hours, to obtain a brownish yellow reaction liquid. Add 2M dilute hydrochloric acid dropwise to adjust the pH of the reaction solution to 5-6, and the color of the reaction solution becomes lighter. Solvent acetonitrile was removed by distillation under reduced pressure, the yellow organic phase was extracted with dichloromethane, dried over anhydrous sodium sulfate, and dichloromethane was removed by distillation under reduced pressure to obtain a crude product, which was subjected to column chromatography (ethyl acetate:petroleum ether) =1:5) separated and purified to obtain 199.6 mg of white solid with a total yield of 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com