Nonmagnetic biomedical implant material and preparation method thereof
A technology for biomedical and implant materials, applied in the field of medical implant materials and their preparation, can solve problems such as slow degradation rate, and achieve the effects of short production cycle, effective and convenient degradation rate, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Single-phase austenitic steel is selected, and its chemical composition mass percentage is: C: 0.6%, Mn: 13%, Si: 0.5%, Ca: 0.5%, Zn: 0.5%, Ag: 0.5%, S: 0.016 %, P: 0.022%, the rest is Fe. The single-phase austenitic steel with the above composition is subjected to three passes of liquid nitrogen cold rolling. Before each pass, the single-phase austenitic steel is placed in liquid nitrogen for 10 minutes. The ratio is: 10:1. The reductions in the three rolling passes were 10%, 8%, and 2% respectively, and the total reduction was 20%. Then perform secondary annealing treatment: heat up to 200°C at a rate of 5°C / min, hold for 60 minutes, and cool with the furnace. After the single-phase austenitic steel returns to room temperature, heat up to 600°C at a rate of 30°C / min. Keeping the temperature for 20 minutes and cooling with the furnace, the non-magnet-based biomedical implant material is prepared.
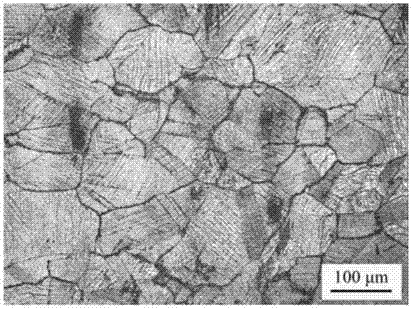
[0019] Depend on figure 1 It can be seen that the single-phase aust...
Embodiment 2
[0021] Single-phase austenitic steel is selected, and its chemical composition mass percentage is: C: 1.5%, Mn: 35%, Si: 0.6%, Ca: 2%, Zn: 2%, Ag: 2%, S: 0.020 %, P: 0.025%, the rest is Fe. The single-phase austenitic steel with the above composition is subjected to three passes of liquid nitrogen cold rolling. The ratio is: 50:1. The reductions in the three rolling passes are respectively 20%, 15%, and 5%, and the total reductions are 40%. Then perform secondary annealing treatment: heat up to 300°C at a rate of 40°C / min, hold for 180 minutes, and cool with the furnace. After the single-phase austenitic steel returns to room temperature, heat up to 800°C at a rate of 40°C / min. Keeping the temperature for 40 minutes and cooling with the furnace, the non-magnet-based biomedical implant material is prepared.
[0022] Depend on figure 2 It can be seen that the corrosion rate of the single-phase austenitic steel after 40% liquid nitrogen cold rolling and secondary annealing i...
Embodiment 3
[0024] Single-phase austenitic steel is selected, and its chemical composition mass percentage is: C: 1.0%, Mn: 25%, Si: 0.55%, Ca: 1%, Zn: 1%, Ag: 1%, S: 0.018 %, P: 0.023%, the rest is Fe. The single-phase austenitic steel with the above composition is subjected to three passes of liquid nitrogen cold rolling. The ratio is: 30:1. The reductions in the three rolling passes were 15%, 10%, and 4% respectively, and the total reduction was 29%. Then perform secondary annealing treatment: heat up to 250°C at a rate of 20°C / min, hold for 100 minutes, and cool with the furnace. After the single-phase austenitic steel returns to room temperature, heat up to 700°C at a rate of 35°C / min. Keeping the temperature for 25 minutes and cooling with the furnace, the non-magnet-based biomedical implant material is prepared.
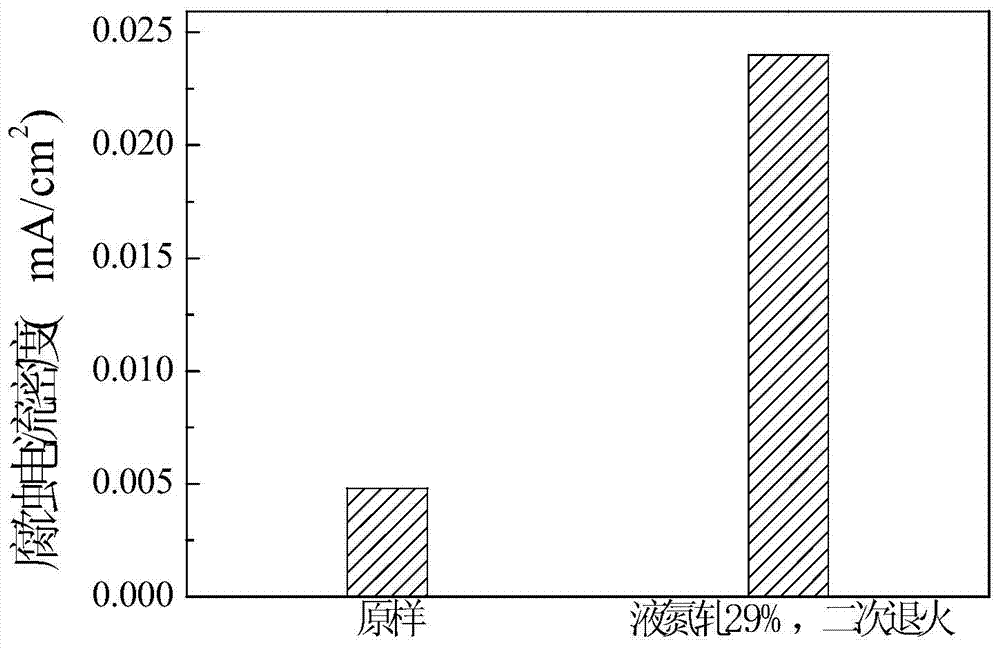
[0025] Depend on image 3 It can be seen that the corrosion current density of single-phase austenitic steel after 29% liquid nitrogen cold rolling and secondary anne...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com