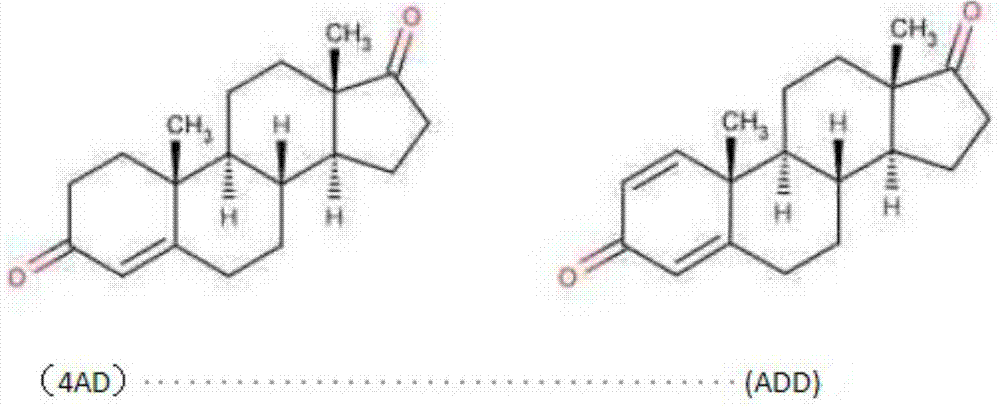
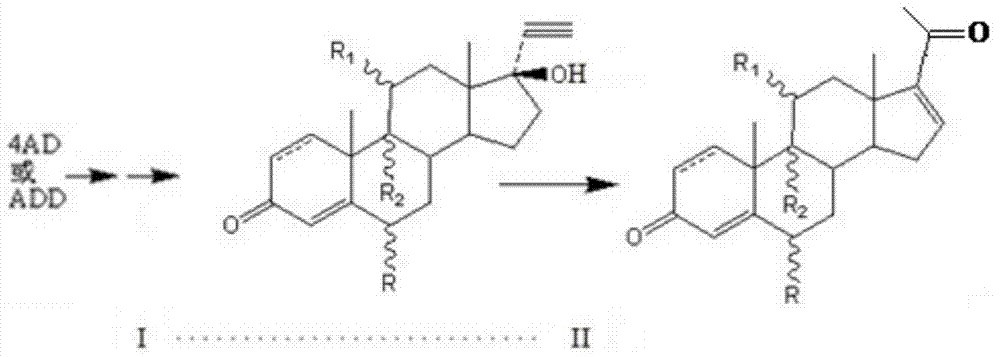
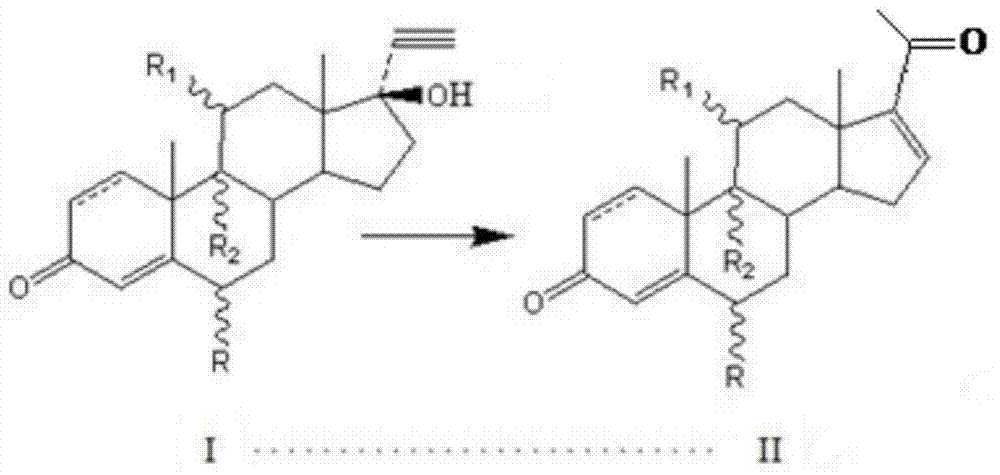
Preparation method for pregna-16-ene-3, 20-dione steroid derivative
A technology of diketone steroids and derivatives, which is applied in the field of steroid synthesis, can solve the problems of being unsuitable for industrial production, cumbersome, and many impurities, and achieve the effects of environmental protection and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0044] Put 700L of carbon tetrachloride into a clean and dry 1000L reaction tank, then add 6.63kg of 15% phosphorus pentoxide-methanesulfonic acid and stir well at room temperature. Then, 3.28 Kg of 11β-hydroxy-17α-ethynyl-17β-hydroxy-4-ene-androst-3-one was added. Steam was heated to 60°C, and the reaction was timed for 1h. TLC detected that the raw material disappeared, cooled to room temperature and filtered, concentrated the filtrate to nearly dryness, rinsed with 500 L of ice water, stirred and cooled to room temperature. Throw material. 2.79 Kg of 11β-hydroxy-4,16-diene-pregna-3,20-dione was obtained with a molar yield of 85% and an HPLC content of 90%.
Embodiment 1-2
[0046] Put 700L of carbon tetrachloride into a clean and dry 1000L reaction tank, then add 6.17kg of 7% phosphorus pentoxide-methanesulfonic acid and stir well at room temperature. Then, 3.28 Kg of 11β-hydroxy-17α-ethynyl-17β-hydroxy-4-ene-androst-3-one was added. Heat the steam to 60°C, and time the reaction for 2h. TLC detected the disappearance of the raw material, cooled to room temperature and filtered, concentrated the filtrate to nearly dryness, rinsed with 300 L of ice water, stirred and cooled to room temperature. Throw material. 2.95 Kg of 11β-hydroxy-4,16-diene-pregna-3,20-dione was obtained with a molar yield of 90% and an HPLC content of 93%.
Embodiment 1-3
[0048] Put 700L of carbon tetrachloride into a clean and dry 1000L reaction tank, then add 6.05kg of 5% phosphorus pentoxide-methanesulfonic acid and stir well at room temperature. Then, 3.28 Kg of 11β-hydroxy-17α-ethynyl-17β-hydroxy-4-ene-androst-3-one was added. Steam was heated to 60°C, and the reaction was timed for 1h. TLC detected that the raw material disappeared, cooled to room temperature and filtered, concentrated the filtrate to nearly dryness, rinsed with 500 L of ice water, stirred and cooled to room temperature. Throw material. 2.79Kg of 11β-hydroxy-4,16-diene-pregna-3,20-dione was obtained with a molar yield of 85% and an HPLC content of 89%.
[0049] Example 2 Preparation of 11α-hydroxy-4,16-diene-pregna-3,20- Diketone, the content of starter is 93% in the present embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com