Application of promoter optimized lentiviral genetically modified T cells in oncotherapy
A promoter and recombinant lentivirus technology, which is applied in the fourth-generation specific anti-tumor immunotherapy technology field, can solve the problems of low efficiency, insufficient expression, and lagging gene modification technology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Optimization of the promoter of the lentiviral expression system
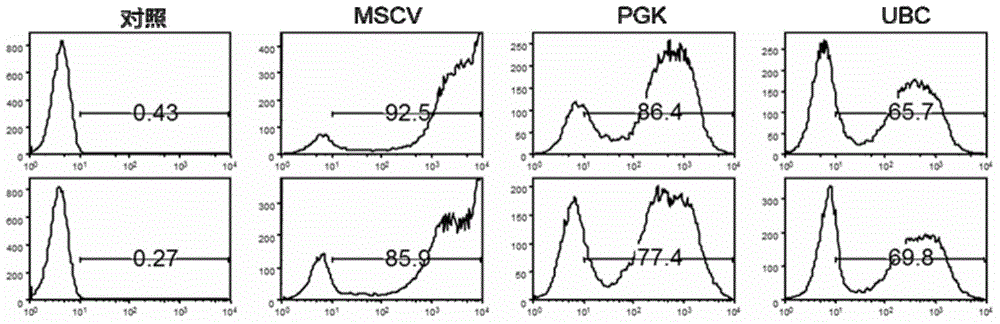
[0039] 1) Packaging and titer determination of recombinant lentivirus
[0040] Culture 293FT cells. One day before transfection, after adjusting the cell density with DMEM medium containing 10% fetal bovine serum, inoculate 25×10 cells per 15 cm cell culture dish. 6 293FT cells into a cell culture dish at 37 °C, 5% CO 2 Cultivate in an incubator, and after 16h-24h, the cell density can be used for transfection when the cell density grows to 80%-90%. On the day of transfection, the medium was replaced with a complete medium (DMEM+10% FBS) without antibiotics (P / S). The LVV-MSCV-GFP, LVV-CMV-GFP, LVV-EF1a-GFP, LVV-PGK-GFP, LVV-UBC-GFP lentiviral backbones were co-transfected with the other three packaging plasmids into 293FT cells, and Lipofectamine 2000 (Invitrogen) as the medium. After culturing for 6 hours, discard the medium, wash with PBS 3 times and replace with 20 ml of fresh complete...
Embodiment 2
[0043] Example 2: The recombinant lentiviral vectors carrying the murine TCRα and β chain genes that specifically recognize human melanoma-associated antigen gp100 (154-162) were transfected into peripheral blood autologous lymphocytes using molecular biology techniques to make the recombinant TCR is expressed in T lymphocytes to achieve the purpose of killing tumors efficiently. A lentiviral vector with an optimized promoter was used to construct a TCR expression vector containing a self-cleaving 2A peptide, Furin and a spacer sequence (see the attached table for the sequence).
[0044] 1) Optimal construction of 2A peptide, furin and spacer sequences expressing the two chains of TCR
[0045] In order to realize the co-expression of TCRα and β chain genes in the same expression vector, the present invention adopts to add 2A peptide with self-cleaving function between TCRα and β chain, and the 2A peptide adopted can be F2A (foot-and-mouth disease virus), E2A (equine Rhinitis ...
Embodiment 3
[0063] Example 3: Using MSCV lentiviral vectors packaged with chimeric antigen receptors (chimeric antigen receptors, CARs), T and NK cells can be genetically modified to express single-chain antibodies, through their specific recognition of tumor cell surface antigens, and Dual activation of T and NK cells through intracellular CD3zeta and 41-BB can not only prolong the survival time of cells, but also enhance their ability to clear tumors, and effectively kill a variety of tumors in vivo and in vitro. The present invention constructs a CAR vector through an anti-Her2 / neu single-chain antibody, genetically modifies human T and NK cells through an MSCV-optimized lentivirus system, and enables them to acquire the ability to specifically kill tumors ( Figure 7 , 8 ,9). In the in vitro cell killing test, CAR-engineered lymphocytes can significantly improve the recognition of T cells to HLA-A2+ corresponding tumor cells, and release IFN-γ, a marker factor of T cell activation. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com