Cell strain for producing goatpox vaccine
A goat pox and cell line technology, applied in the direction of microorganism-based methods, animal cells, vertebrate cells, etc., can solve the problems of lamb infection, high cost of lamb, limited number of cells, etc., and achieve the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]Comparison of Proliferation Ability of Goat Pox Virus AV41 Strain in Different Cells
[0045] (1) Virus strains and cells: goat pox virus chicken embryo attenuated strain AV41 (unpurified) was purchased from China Veterinary Drug Administration, hamster kidney cells (BHK21), pig testicular cells (ST), bovine embryonic kidney cells (MDBK) 3 kinds Cells were purchased from China Veterinary Drug Administration, and VeroB10 was screened and preserved in our laboratory. The VeroB10 cell clone is preserved in the General Microbiology Center of China Microbiological Culture Collection Management Committee, and the preservation number is CGMCC No.9705.
[0046] (2) Cell growth medium: (volume percentage) 92% DMEM, 8% newborn bovine serum, pH 7.0; cell maintenance medium: (volume percentage) 98% DMEM, 2% newborn bovine serum, The pH is 7.3.
[0047] (3) Virus proliferation: Prepare the above four kinds of cell monolayers according to the conventional method, inoculate goat pox ...
Embodiment 2
[0052] Preparation of Goat Pox Live Vaccine Using Two Passage Cells
[0053] (1) Virus strains and cells: The goat pox virus AV41 strain propagated for 1 generation on BHK21 and VeroB10 respectively was used as seed virus, and BHK21 and VeroB10 cells were used as production cells.
[0054] (2) Cell subculture and observation: After BHK21 and VeroB10 cells were digested and dispersed with 0.25% trypsin digestion solution, BHK21 cells were subcultured at a ratio of 1:4, and VeroB10 cells were subcultured at a ratio of 1:3. Cell growth medium was added and continued at 37°C. Culture until the cells grow into a monolayer.
[0055] (3) Virus inoculation and harvesting: Take BHK21 and VeroB10 cells that have formed a good monolayer, discard the cell growth medium, inoculate goat pox virus AV41 strain, add maintenance solution after adsorption for 1 hour, continue to culture at 37 °C, and wait for 75 Harvest the virus when the % cell is pathological, and the prepared cytovenom is a ...
Embodiment 3
[0060] Application Evaluation of Goat Pox Live Vaccine Prepared by Different Production Methods
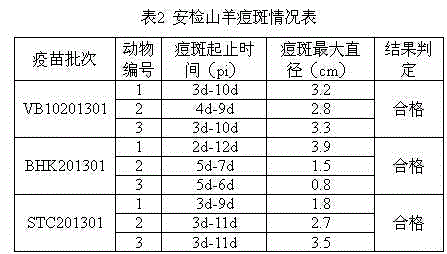
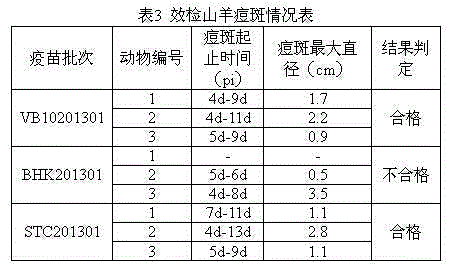
[0061] (1) Experimental vaccine: goat pox vaccine prepared with two passaged cells, batch numbers VB10201301 and BHK201301 respectively; goat pox vaccine prepared with primary lamb testicular cells, batch number STC201301, all of which have passed sterility testing and specificity The inspection, exogenous virus inspection and mycoplasma inspection are qualified, and the virus content in each vaccine is not less than 10 3.5 TCID 50 .
[0062] (2) Test animals: 18 healthy goats for testing.
[0063] (3) Safety inspection: Dilute the vaccine with sterilized physiological saline so that the virus content per ml is 4×10 3.5 TCID 50 , 3 goats were injected intradermally in the chest and abdomen, each injected 2 points, 0.5ml per point, and observed for 15 days. The 3 goats vaccinated by each batch of vaccines had no obvious fluctuations in body temperature within 15 days after i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com