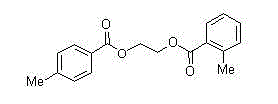
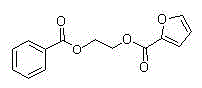
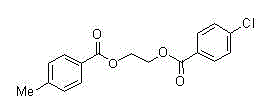
Synthetic method of 1, 2-diol dicarboxylic ester
A technology of carboxylic acid and compound, applied in the field of preparation of 1,2-diol dicarboxylate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1, use 2,3-dichloro-5,6-dicyano-1,4-benzoquinone to promote cyclic acetal and carboxylic acid to synthesize diester and detect the influence of different solvents on the esterification reaction (expressed in formula Show I-1 compound as an example).
[0014] 2,3-dichloro-5,6-dicyano-1,4-p-benzoquinone (1.2 mmol), benzoic acid (1 mmol), different reaction solvents (2 mL) (nitromethane, acetonitrile, Ethyl acetate, chloroform, 1,2-dichloroethane, dichloromethane, benzene) and benzaldehyde ethylene acetal (2 mmol) were added into the sealed tube, and placed directly at 80°C for 10 hours. Add saturated aqueous sodium bicarbonate solution to the reaction solution, extract with ethyl acetate, separate the esterification product by silica gel column chromatography, and calculate the separation yield as shown in Table 1, wherein, the yield of the target product ester in dichloromethane is obtained The highest value, 90%, sets the best solvent as dichloromethane.
...
Embodiment 2
[0018] Embodiment 2, the influence of reaction temperature on the dehydrogenation coupling reaction of the present invention.
[0019] Except for different reaction temperatures (100°C, 80°C, 60°C, 40°C), other reaction conditions were the same as in Example 1, and the influence of reaction temperature on the coupling reaction yield was detected. After the reaction, the measurement results of the separation yield of the target ester are shown in Table 2, which shows that there is an optimum value for the yield of the coupling reaction with the change of the reaction temperature, and the optimum reaction temperature is set at 80°C.
[0020] Reaction temperature (°C) 100 80 60 40 Separation yield (%) 89 90 78 57
Embodiment 3
[0021] Example 3, the effect of the ratio of cyclic acetal to carboxylic acid on the dehydrogenation coupling reaction of the present invention.
[0022] Except the molar ratio of cyclic acetal and carboxylic acid is different (1:1, 2:1, 3:1, 4:1, 10; 1), other reaction conditions are the same as in Example 1, and the detection of cyclic acetal and carboxylic acid Effect of molar ratio of acid on yield of coupling reaction. After the reaction finishes, the separation yield measurement result of target ester is as shown in table 3, shows that along with the change of the molar ratio of cyclic acetal and carboxylic acid, there is an optimal value in the yield of coupling reaction, and cyclic acetal and The optimal molar ratio of carboxylic acid is 2:1.
[0023] Cyclic acetal / carboxylic acid ratio (mol / mol) 1 2 3 4 10 Separation yield (%) 68 90 90 90 85
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com