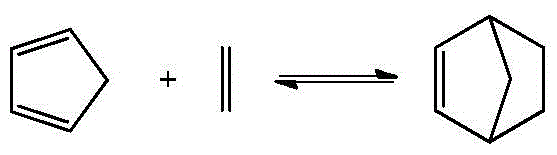Method for synthesizing norbornene by microchannel reactor
A micro-channel reactor, norbornene technology, applied in the direction of addition of unsaturated hydrocarbons to hydrocarbons, organic chemistry, etc., can solve the problem of increasing equipment investment and operating costs, and has not yet seen norbornene synthesis and norbornene conversion rate. Low problems, to avoid the generation of reaction hotspots, inhibit side reactions, improve safety and synthesis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
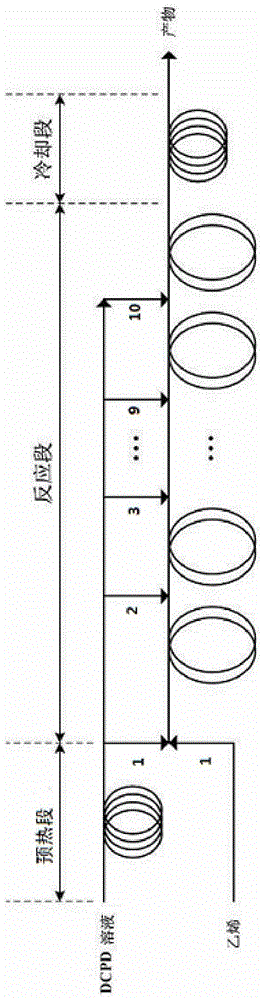
[0051] Prepare a dicyclopentadiene toluene solution with a mass fraction of 40 wt%, wherein the mass ratio of cyclopentadiene to dicyclopentadiene is 1:19. That is, dicyclopentadiene toluene solution is made up of cyclopentadiene and dicyclopentadiene of 40% mass total concentration and toluene as balance (that is, 60%), wherein cyclopentadiene and dicyclopentadiene mass The ratio is 1:19.
[0052] Under normal temperature conditions, ethylene is directly transported to the microchannel reaction device from the first side line feed port, and the dicyclopentadiene toluene solution is fed from the first side line feed port, the third side line feed port and the fifth side line feed port respectively. Be divided into three equal quantities and be transported in the tubular reaction device, ethylene volume flow is 4.00ml / min, and the volume flow of total dicyclopentadiene toluene solution is 0.45ml / min, the mol ratio of ethylene and dicyclopentadiene toluene ( The dicyclopentadie...
Embodiment 2
[0060] Prepare a dicyclopentadiene toluene solution with a mass fraction of 10 wt%, wherein the mass ratio of cyclopentadiene to dicyclopentadiene is 1:19. That is, the dicyclopentadiene toluene solution is composed of cyclopentadiene and dicyclopentadiene with a mass total concentration of 10% and toluene as a balance (that is, 90%), wherein cyclopentadiene and dicyclopentadiene mass The ratio is 1:19.
[0061] Under normal temperature conditions, ethylene is directly transported to the microchannel reaction device from the first side line feed port, and the dicyclopentadiene toluene solution is fed from the first side line feed port, the second side line feed port and the third side line feed port respectively. It is divided into three equal parts and transported in the tubular reaction device, the ethylene volume flow rate is 6.21ml / min, the volume flow rate of the total dicyclopentadiene toluene solution is 1.76ml / min, and the mol ratio of ethylene to dicyclopentadiene tol...
Embodiment 3
[0063] Prepare a dicyclopentadiene toluene solution with a mass fraction of 40 wt%, wherein the mass ratio of cyclopentadiene to dicyclopentadiene is 1:19.
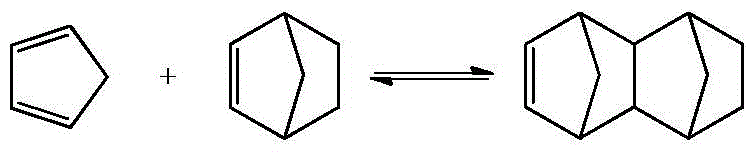
[0064] Under normal temperature conditions, ethylene is directly transported from the first side feed port to the microchannel reaction device, and the dicyclopentadiene toluene solution is fed from the first side feed port, the third side feed port, and the fifth side feed port respectively. , The seventh side line feed port and the ninth side line feed port are divided into five equal parts and sent to the tubular reaction device, the ethylene volume flow rate is 1.85ml / min, and the total dicyclopentadiene toluene solution volume flow rate is 0.31ml / min min, the molar ratio of ethylene to dicyclopentadiene toluene solution is 1.0. Dicyclopentadiene is partially or completely decomposed into cyclopentadiene by heating and pre-decomposing in the preheating section before entering the reaction section. The preheating tempe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com