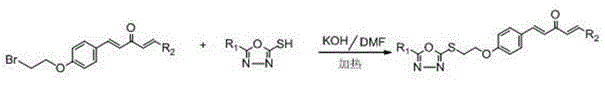
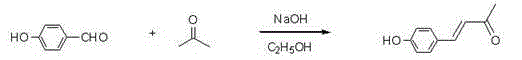Preparation method and use of pentadiene ketone compound containing 1,3,4-oxadiazole sulfo-ethyoxyl
A technology of oxadiazole thioethoxy and pentadienone, which is applied in the field of chemistry and can solve problems such as no anti-plant virus activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: (1 E ,4 E )-1-(4-fluoro-phenyl)-5-(4-((5-(4-fluorophenyl)-1,3,4-oxadiazole)-2-thio)ethoxyphenyl )-1,4-Pentadien-3-one synthesis:
[0109] (1) Preparation of methyl p-fluorobenzoate
[0110] Put 0.1 mol of p-fluorobenzoic acid and 50 mL of anhydrous methanol into a 100 mL three-neck flask, slowly add 0.1 mol of concentrated sulfuric acid dropwise at room temperature, and then raise the temperature to reflux, and the reaction was completed for 8 hours. Recover methanol under reduced pressure, add 50 mL of water, wash the solution with saturated sodium bicarbonate solution until neutral, and recrystallize with methanol after extraction.
[0111] (2) Preparation of p-fluorobenzoic hydrazide
[0112] Put 0.05 mol of methyl p-fluorobenzoate and 50 mL of methanol into a 100 mL three-necked round-bottomed flask, slowly add 80% hydrazine hydrate (0.06 mol) at room temperature, and heat up to reflux for 7 hours to complete the reaction. Methanol was recovered un...
Embodiment 2
[0123] Example 2 :(1 E ,4 E )-1-(4-chloro-phenyl)-5-(4-((5-(4-fluorophenyl)-1,3,4-oxadiazole)-2-thio)ethoxyphenyl )-1,4-Pentadien-3-one synthesis:
[0124] Steps (1)-(4) are the same as in Example 1;
[0125] The difference between step (5) and embodiment 1 step (5) is: adding 4-chlorobenzaldehyde instead of 4-fluorobenzaldehyde;
[0126] The difference between step (6) and embodiment 1 step (6) is: add (1 E ,4 E )-1-(4-chlorophenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one instead of (1 E ,4 E )-1-(4-fluorophenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one;
[0127] The difference between step (7) and embodiment 1 step (7) is: add (1 E ,4 E )-1-(4-(2-bromoethoxy)-phenyl)-5-(4-chlorophenyl)-1,4-pentadien-3-one instead of (1 E ,4 E )-1-(4-(2-bromoethoxy)-phenyl)-5-(4-fluorophenyl)-1,4-pentadien-3-one.
Embodiment 3
[0128] Example 3 :(1 E ,4 E )-1-(4-bromo-phenyl)-5-(4-((5-(4-fluorophenyl)-1,3,4-oxadiazole)-2-thio)ethoxyphenyl Synthesis of )-1,4-pentadien-3-one
[0129] Steps (1)-(4) are the same as in Example 1;
[0130] The difference between step (5) and embodiment 1 step (5) is: adding 4-bromobenzaldehyde instead of 4-fluorobenzaldehyde;
[0131] The difference between step (6) and embodiment 1 step (6) is: add (1 E ,4 E )-1-(4-bromophenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one instead of (1 E ,4 E )-1-(4-fluorophenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one;
[0132] The difference between step (7) and embodiment 1 step (7) is: add (1 E ,4 E )-1-(4-(2-bromoethoxy)-phenyl)-5-(4-bromophenyl)-1,4-pentadien-3-one instead of (1 E ,4 E )-1-(4-(2-bromoethoxy)-phenyl)-5-(4-fluorophenyl)-1,4-pentadien-3-one.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com