Recombinant escherichia coli for preparing (S)-4-chlorine-3-hydroxyl ethyl butyrate by adopting asymmetric transformation and application of recombinant escherichia coli
A technology of recombinant Escherichia coli and ethyl hydroxybutyrate, applied in the field of enzyme catalysis, can solve the problems of large pollution, low yield, high energy consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
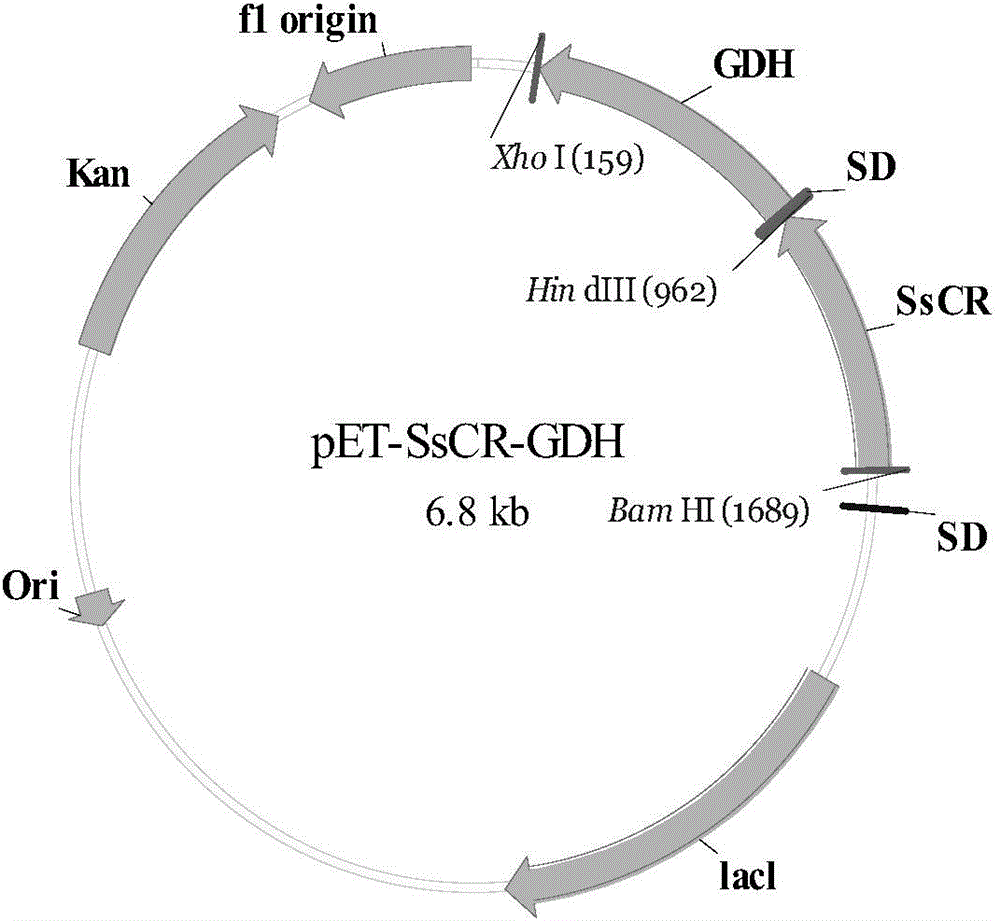
[0027] Embodiment 1: Construction of recombinant escherichia coli
[0028] 1. Acquisition of carbonyl reductase gene:
[0029] Cyanobacteria Synechocystis sp.PCC 6803 (purchased from Pasteur Culture Collection of Cyanobacteria), medium Zobell 2216E (g L -1 ): peptone 5g, yeast extract 1g, ferric phosphate 0.1g, aged sea water 1000mL, pH 7.6.
[0030] The cyanobacteria Synechocystis sp.PCC 6803 was inoculated in 4 mL of Zobell 2216E liquid medium at 25°C to the logarithmic growth phase, and the genome was extracted using a genomic DNA extraction kit (Bacterial Genome Extraction Kit from Shanghai Jierui Company).
[0031] The primers used to construct the expression vectors are provided with enzyme cutting sites, and the primer sequences are as follows:
[0032]The upstream primer (SrCR-F contains Bam HI) is: AACGCGGATCCATGTTAAGTCTT GGTTTGGAAG, the downstream primer (SrCR-R contains HindⅢ) is: AACCCAAAGCTTAGGTGTGGTGGGCCCCATTT, and all primers are synthesized by Shanghai Jierui...
Embodiment 2
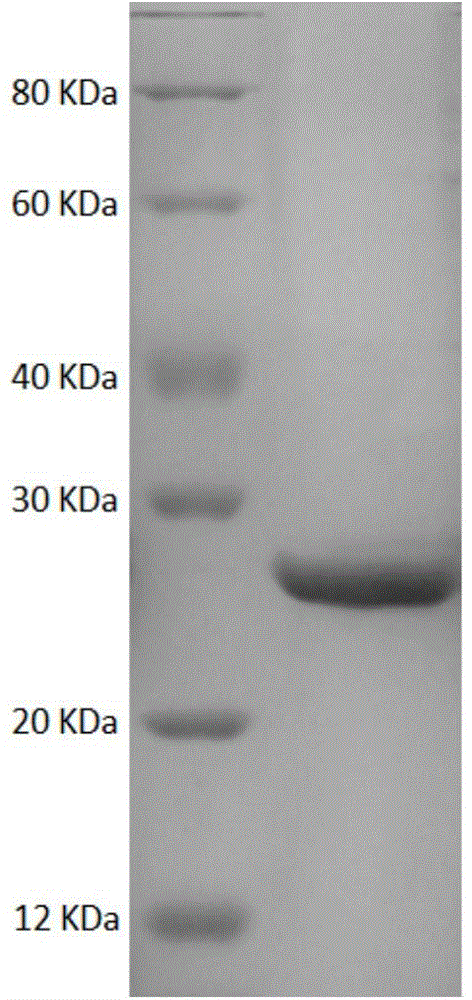
[0045] Example 2: Obtaining whole cells of recombinant Escherichia coli E.coli (pET28a-SrCR-GDH)
[0046] The transformants obtained in Example 1 were inoculated into LB liquid medium containing 50 μg / ml kanamycin resistance, cultured at 37° C. for 12 h, and then inoculated into fresh containing In 50ug / ml kanamycin-resistant LB liquid medium, culture at 37°C until the cell concentration OD600 is about 0.5, then add IPTG with a final concentration of 0.1mM to the LB liquid medium, and induce culture at 20°C for 20 hours, Centrifuge the culture solution at 4°C and 5000rpm for 6min, discard the supernatant, and collect the precipitate, which is the recombinant Escherichia coli (pET28a-SrCR-GDH) wet bacteria. Freeze-dried cells were obtained after the wet cells were freeze-dried for 4 hours. Centrifuge the precipitate after breaking the wet bacteria, take the supernatant and purify it with a nickel column, elute the impurity protein under 20mM imidazole, and elute the target pr...
Embodiment 3
[0047] Example 3: Application of the recombinant Escherichia coli in the preparation of (S)-CHBE
[0048] The freeze-dried thallus obtained in Example 2 was used as a catalyst. Weigh 0.5g freeze-dried bacteria and suspend in 25mL Trish-Hcl (100mM), add glucose 35mM, COBE 20mM, NADP 0.2mM, 30°C, react for 10min, the final substrate conversion rate reaches 100%, and the product optical purity e.e % is 99.4%.
[0049] The freeze-dried thallus obtained in Example 2 was used as a catalyst. Weigh 0.5g freeze-dried bacteria and suspend in 25mL Trish-Hcl (100mM), add glucose 600mM, COBE 400mM, NADP 0.2mM, 30℃, after 30min, the conversion rate of the final substrate reaches 100%, and the optical purity of the product is e.e. % is 99.3%.
[0050] The freeze-dried thallus obtained in Example 2 was used as a catalyst. Weigh 0.5g freeze-dried bacteria and suspend in 25mL Trish-Hcl (100mM), add glucose 900mM, COBE 600mM, NADP0.2mM, 30℃, after 1h reaction, the final substrate conversion ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com