Linagliptin composition and preparation method thereof
A technology of linagliptin and wet granulation, which is applied in the field of linagliptin pharmaceutical composition and its preparation, can solve the problems of bitter taste of the drug, inconvenient taking of the patient, and reduced compliance of taking the drug, so as to achieve rapid disintegration, fast dissolving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
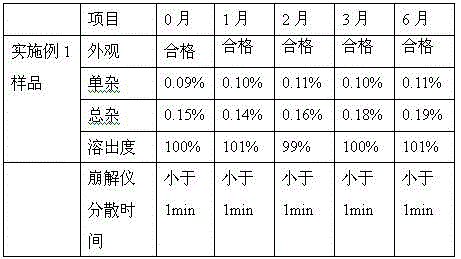
Embodiment 1
[0037] Table 1
[0038]
[0039] Preparation Process:
[0040] 1. Linagliptin, lactose, and crospovidone are mixed in a wet granulation pot,
[0041] 2. Dissolve and disperse povidone and aspartame into 95% ethanol solution, add to granulation pot for granulation to prepare granules,
[0042] 3. Dry the granules obtained in step "2", granulate,
[0043] 4. Mix the particles with silica,
[0044] 5. Fill the mixed material into the bag.
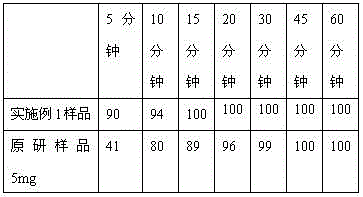
[0045] The granules prepared in Examples 1 and 2, and the original research tablet were tested for dissolution curves. The detection method was: dissolution medium volume: 1000ml, dissolution medium was hydrochloric acid solution of pH 1.0, rotating speed was 50rpm, paddle method. The dissolution profile results are shown in Table 2.
[0046] Table 2
[0047]
Embodiment 2
[0049] table 3
[0050]
[0051] Preparation Process:
[0052] 1. Mix linagliptin, mannitol, lactose, crospovidone, menthol, magnesium stearate,
[0053] 2. The mixed material is rolled and pressed by a dry granulator to obtain a tablet.
[0054] 3. Pulverize the flakes obtained in step "2" to obtain granules,
[0055] 4. Add silicon dioxide and mix it.
[0056] 5. Fill the mixed material into the bag.
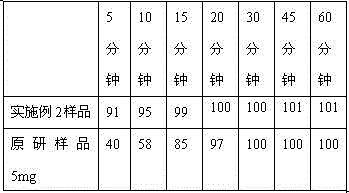
[0057] For the preparation prepared in Examples 3 and 4, the detection of the dissolution curve is carried out, and the detection method is: the volume of the dissolution medium:
[0058] 1000ml, the dissolution medium is hydrochloric acid solution with pH 1.0, the rotation speed is 50rpm, paddle method.
[0059] Table 4
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com