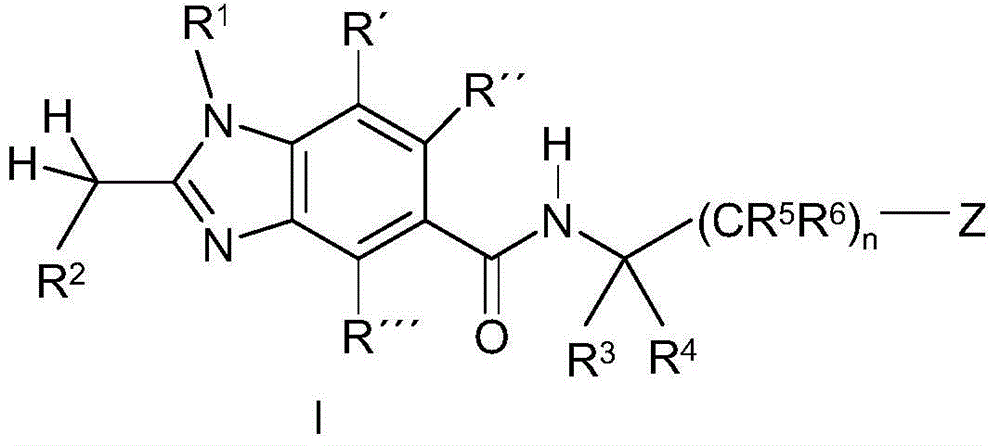
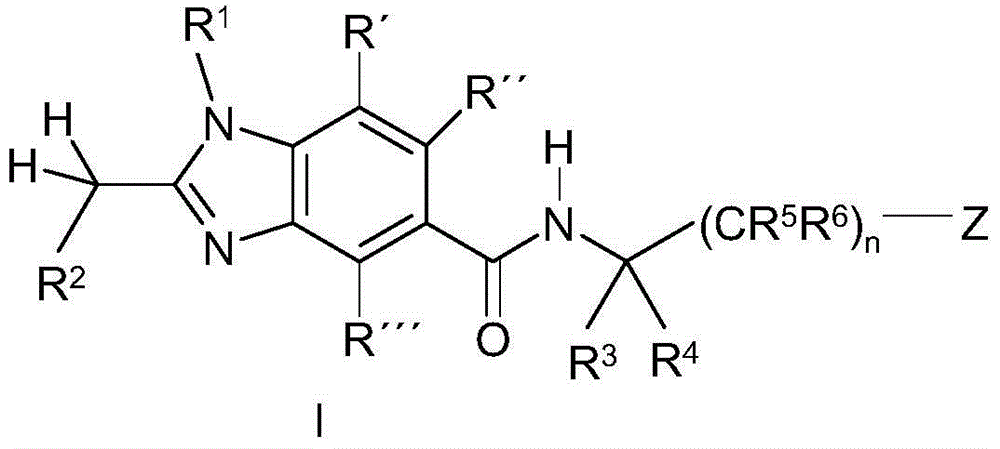
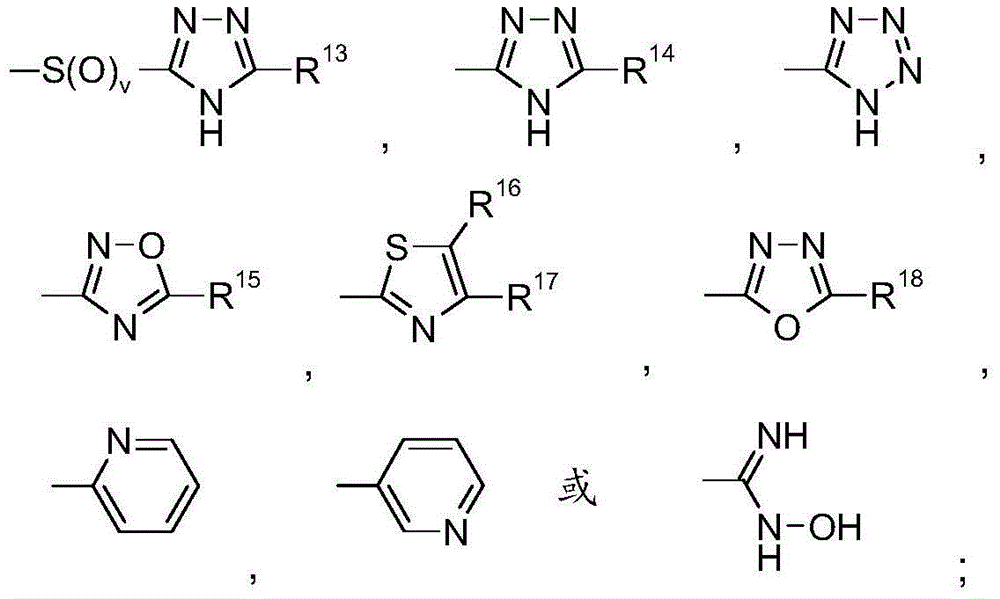
Benzoimidazole-carboxylic acid amide derivatives as APJ receptor modulators
A solvate and phenyl technology, which can be used in drug combinations, medical preparations containing active ingredients, metabolic diseases, etc., and can solve problems such as difficult reconciliation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1948] Example 1: 1-{[1-(1-Ethyl-propyl)-2-thiophen-2-ylmethyl-1H-benzimidazole-5-carbonyl]-amino}-cycloheptanecarboxylic acid
[1949]
[1950] a) Methyl 1-{[1-(1-ethyl-propyl)-2-thiophen-2-ylmethyl-1H-benzimidazole-5-carbonyl]-amino}-cycloheptanecarboxylate
[1951]
[1952] At 0°C, add 160 mg of 1-(1-ethyl-propyl)-2-thiophen-2-ylmethyl-1H-benzimidazole-5-carboxylic acid (the preparation of the intermediate is described below) in 3 ml To a solution in dry DMF was added 73 mg HOAT, 131 mg EDC and 0.16 ml DIPEA. After 15 min 100 mg of methyl 1-amino-cyclopentanecarboxylate hydrochloride and 0.16 ml of DIPEA were added and the reaction mixture was stirred at room temperature for 16 h. The reaction mixture was then poured into water and the pH was adjusted to 3 by adding 2M aqueous hydrochloric acid. The reaction mixture was extracted three times with ethyl acetate. The combined organic phases were washed with saturated aqueous sodium bicarbonate and brine, dried over m...
Embodiment 80
[1977] Example 80: (S)-2-{[1-(1-Ethyl-propyl)-2-(tetrahydro-furan-2-ylmethyl)-1H-benzimidazole-5-carbonyl]- Amino}-4-methyl-pentanoic acid
[1978]
[1979] a)(S)-2-{[1-(1-Ethyl-propyl)-2-(tetrahydro-furan-2-ylmethyl)-1H-benzimidazole-5-carbonyl]-amino} -4-Methyl-pentanoic acid tert-butyl ester
[1980]
[1981] At 0°C, to 55mg 1-(1-ethyl-propyl)-2-(tetrahydro-furan-2-ylmethyl)-1H-benzimidazole-5-carboxylic acid in 1ml dry DMF 26mg HOBT, 37mg EDC and 0.05ml DIPEA were added to the solution. After 15 min 100 mg L leucine tert-butyl ester hydrochloride and 0.05 ml DIPEA were added and the reaction mixture was stirred at room temperature for 16 h. The reaction mixture was then poured into water and the pH was adjusted to 3 by adding 2M aqueous hydrochloric acid. The reaction mixture was extracted three times with ethyl acetate. The combined organic phases were washed with saturated aqueous sodium bicarbonate and brine, dried over magnesium sulfate and concentrated. Ob...
Embodiment 107
[1994] Example 107: (S)-3-{[1-(1-Ethyl-propyl)-2-thiophen-2-ylmethyl-1H-benzimidazole-5-carbonyl]-amino}-5- methyl-hexanoic acid
[1995]
[1996] In a 20 ml scintillation vial, to 300 mg Wang resin (NovaBioChem 70-90 mesh, loading capacity 1.3 mmol / g) in DMF was added 430 mg (S)-3-(9H-fluoren-9-ylmethoxy (carbonylamino)-5-methyl-hexanoic acid, 173 mg DIC and 14 mg DMAP. The reaction mixture was kept at room temperature for 18 h. To deprotect the Fmoc group, 50% piperidine / DMF was added and the reaction mixture was kept at room temperature for 30 min. Thereafter the resin was washed thoroughly with DMF. For amide formation, the resin was reacted with 217 mg 4-fluoro-3-nitrobenzoic acid, 185 mg HOBt and 173 mg DIC in DMF at room temperature for 18 h. In the next step, nucleophilic substitution was achieved by reacting the resin with 680 mg 1-ethyl-propylamine in DMF at room temperature for 24 h. Then pass through with 10ml 1M SnCl 2 The nitro group was reduced in DMF a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com