Hepatocyte growth factor receptor key structural domain fusion protein and application thereof
A technology of hepatocyte growth factor and fusion protein, which is applied in the field of fusion protein, can solve the problem of no anti-C-Met neutralizing antibody drug, etc., and achieve the effect of good application prospect, low cost and simple construction method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Construction of lentiviral expression vector for ED-His fusion protein
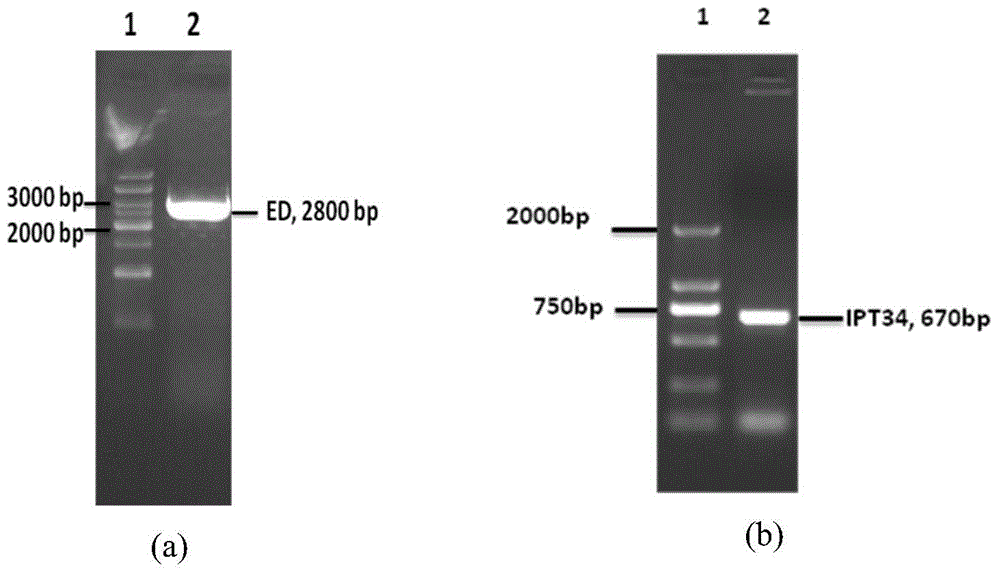
[0027] Using pCR-Blunt-EGFR containing the full-length sequence of human EGFR as a template, primers P1 and P2 (synthesized by Suzhou Jinweizhi Co., Ltd.) were designed and synthesized according to the signal peptide sequence of EGFR gene, and an enzyme cutting site Age I was added to the upstream primer to amplify A 78bp signal peptide was added. Using pCR-Blunt-C-Met containing the full-length human C-Met gene as a template, PCR primers P3 and P4 (synthesized by Suzhou Jinweizhi Co., Ltd.) were designed and synthesized according to the sequence of the extracellular region of the human C-Met gene registered on GenBank, A restriction site SalI and a His tag were added to the downstream primers, and the target fragment ED of about 2800 bp was amplified. Using these two PCR products as templates and using P1 and P4 as primers, a second PCR amplification reaction was performed, and the two PCR produc...
Embodiment 2
[0036] Construction of IPT34-His fusion protein lentiviral expression vector
[0037] Using pCR-Blunt-EGFR containing the full-length sequence of human EGFR as a template, primers P1 and P2 (synthesized by Suzhou Jinweizhi Co., Ltd.) were designed and synthesized according to the signal peptide sequence of EGFR gene. Using pCR-Blunt-C-Met as a template, primers P5 and P6 (synthesized by Suzhou Jinweizhi Co., Ltd.) were designed and synthesized according to the sequence of the IPT34 region of the human C-Met gene. The first PCR amplification reaction was performed separately to obtain two PCR products. Using these two PCR products as templates and using P1 and P6 as primers, a second PCR amplification reaction was performed, and the two PCR products were joined together by overlap extension PCR. The amplified product was first cloned into the pCR-Blunt cloning vector, followed by digestion, ligation and other steps, and finally the lentiviral expression vector pRRL-CMV-IPT34 w...
Embodiment 3
[0040] Expression and purification of recombinant C-Met-ED and C-Met-IPT34 fusion proteins
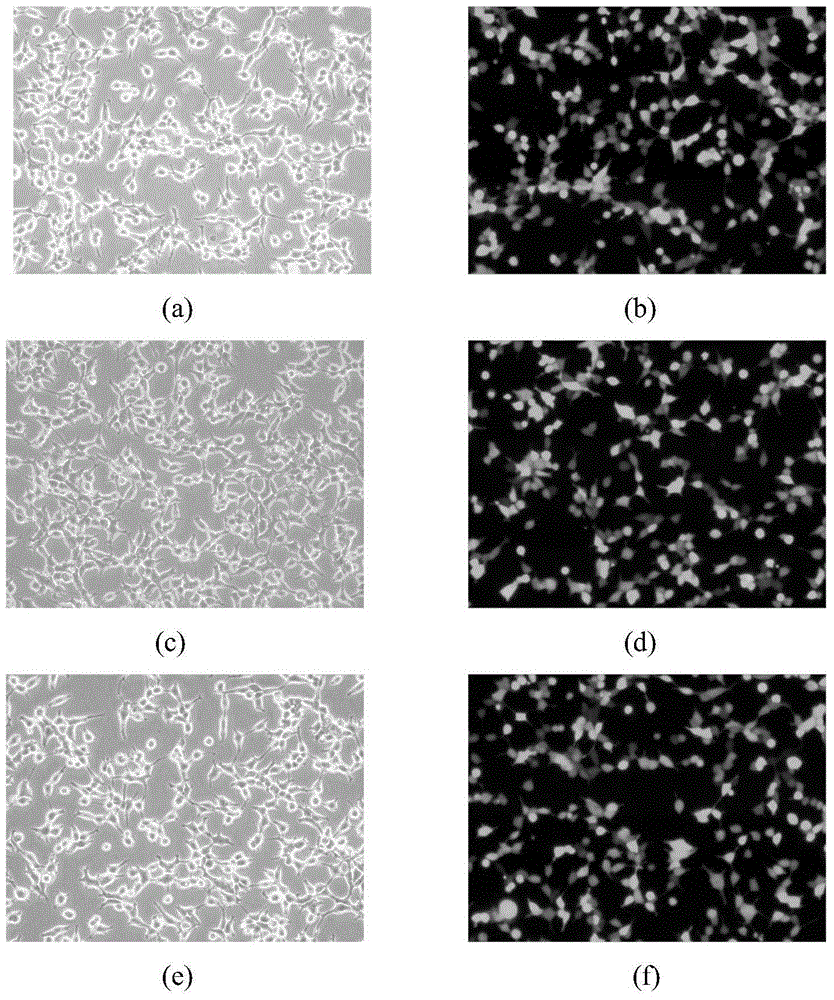
[0041] 293T cells in the logarithmic growth phase were inoculated, and when the cells grew to 50%-60% confluence, the calcium phosphate method was used to mix pRRL-CMV-C-Met-ED with the control vector containing green fluorescent protein (pRRL-CMV- GFP) co-transfected 293T cells to express C-Met-ED protein. Meanwhile, in order to express C-Met-IPT34 protein in 293T cells, pRRL-CMV-C-Met-IPT34 and pRRL-CMV-GFP were co-transfected into 293T cells. 293T cells transfected with pRRL-CMV-GFP were used as positive control, and 293T cells without transfection were used as blank control. After 48 h of cell culture, if image 3 As shown, the cells of the C-Met-ED group, the C-Met-IPT34 group and the positive control group all expressed green fluorescent protein through fluorescence microscope observation, while the cells of the negative control group that were not transfected with the plasmid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com