Double-bond-containing cyclic halamine antibacterial agent precursor as well as preparation method and application thereof
A cyclic halamine and antibacterial agent technology, which is applied in the field of antibacterial materials, can solve the problems of difficult-to-use plastic antibacterial plastics with halamine antibacterial agents, long-term use performance decline, and degradation of halamine precursors, and achieve excellent antibacterial performance and production costs. The effect of low, short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
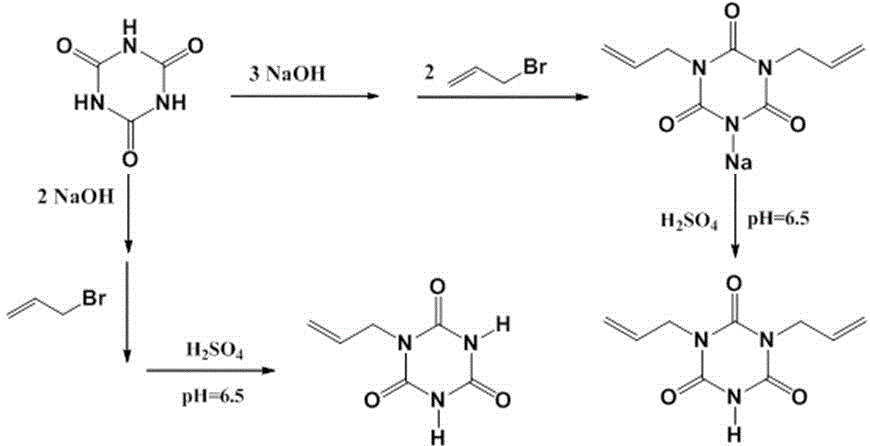
[0035] Example 1 : Synthesis of halamine antibacterial agent 1,3-diallyl-S-triazinetrione.
[0036] Weigh 5.16 g (0.04 mol) of cyanuric acid and place it in a 250 mL flask, add 100 mL of deionized water, stir evenly with a glass rod, then add 4.8 g of sodium hydroxide, continue stirring until the cyanuric acid is completely dissolved, slowly Add 9.60 g (0.08 mol) of allyl bromide dropwise, stir the reaction at 25°C for 10 h, and adjust the pH value of the reaction solution to 6.5 with dilute hydrochloric acid; a white solid powder is precipitated, washed with deionized water to obtain 1,3-di Allyl-S-triazinetrione, yield 76%.
Embodiment 2
[0037] Example 2 : Synthesis of halamine antibacterial agent 1-allyl-S-triazinetrione.
[0038] Weigh 5.16 g (0.04 mol) of cyanuric acid and place it in a 250 mL flask, add 100 mL of deionized water, stir evenly with a glass rod, then add 4.5 g of potassium hydroxide, continue stirring until the cyanuric acid is completely dissolved, slowly Add 4.80 g (0.04 mol) of allyl bromide dropwise, stir the reaction at 25°C for 10 h, and adjust the pH value of the reaction solution to 7.0 with dilute sulfuric acid; a white solid powder is precipitated and washed with deionized water to obtain 1-allyl bromide -S-triazinetrione, yield 80%.
Embodiment 3
[0039] Example 3 : Synthesis of halamine antibacterial agent 1,3-diallyl-S-triazinetrione. (Using allyl chloride as raw material)
[0040]Weigh 5.16 g (0.04 mol) of cyanuric acid and place it in a 250 mL flask, add 100 mL of deionized water, stir evenly with a glass rod, then add about 6.0 g of sodium carbonate, continue stirring until the cyanuric acid is completely dissolved, slowly Add 6.10 g (0.08 mol) allyl chloride dropwise, stir the reaction at 20°C for 16 h, and adjust the pH value of the reaction solution to 6.5 with dilute hydrochloric acid; white solid powder is precipitated, washed with deionized water to obtain 1,3-di Allyl-S-triazinetrione, yield 68%.
[0041] Application examples of cyclic haloamine antibacterial agents containing double bonds:
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com