Crystal form of azetidinone compound and preparation method thereof
A compound and crystal form technology, applied in the field of chemistry, can solve the problem of no crystal form of the compound of formula A, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1: (3R, 4S)-4-(4-hydroxyphenyl)-3-[3-(4-fluorophenyl)-4-hydroxybutyl-2(Z)-ene]-1-( Preparation of 4-fluorophenyl)-2-azetidinone (compound of formula A)
[0132] The preparation of the compound of formula A refers to the method of WO2011017907A1, specifically as follows:
[0133]
[0134] Step 1: Add 32.5 mmoles of compound II (Formula Z), 250 mL of methanol, and 4.89 g (35.8 mmoles) of potassium carbonate to a 500 mL reaction flask, and stir at room temperature for 30 minutes. After the reaction was completed, it was extracted three times with ethyl acetate (300 mL×3), and the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated to dryness, and kept for later use.
[0135] Step 2: Dissolve the product of step 1 in 200 mL of tetrahydrofuran, adjust the pH to about 1 with 6 mol / L hydrochloric acid, stir and react at room temperature for 30 minutes, then extract 3 times with ethyl acetate (250 mL×3), combi...
Embodiment 2
[0137] Embodiment 2: the preparation of crystal form I
[0138] At room temperature, 1 g of the compound of formula A was dissolved in 1 mL of methanol to prepare its saturated solution, and then 10 mL of water was added to the saturated solution at one time, and the obtained solid was filtered out immediately, and dried in a vacuum drying oven (the drying temperature was 35 ~40° C.), the dried solid is the crystal form I, the yield is 0.73 g, and the yield is 73%.
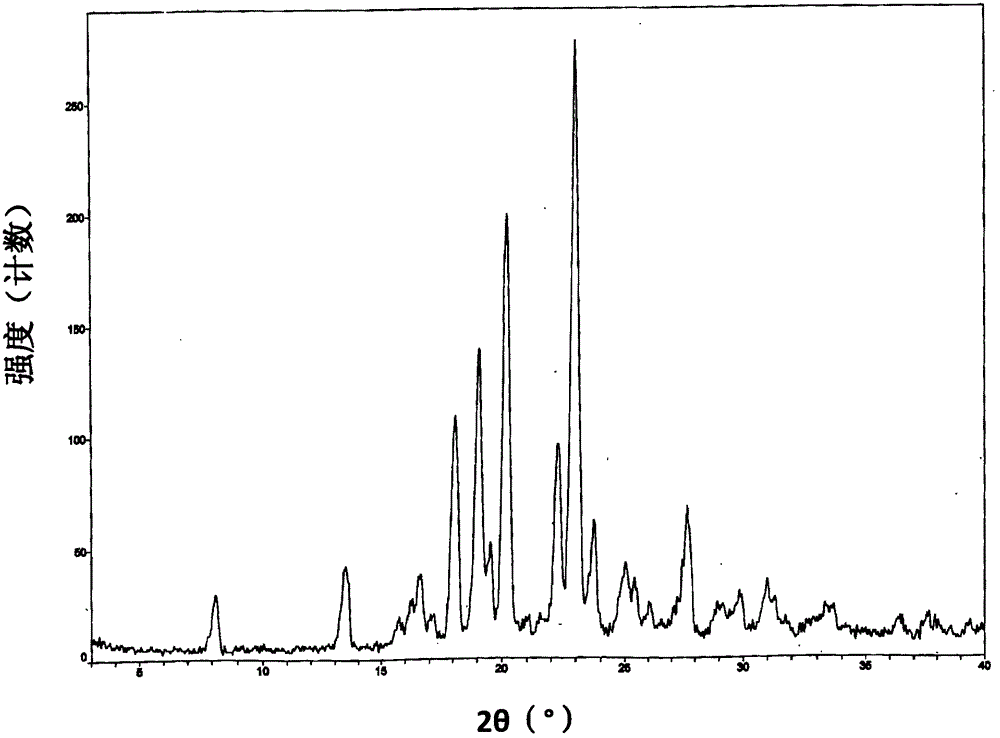
[0139] Its XRPD pattern is as follows figure 1 shown.
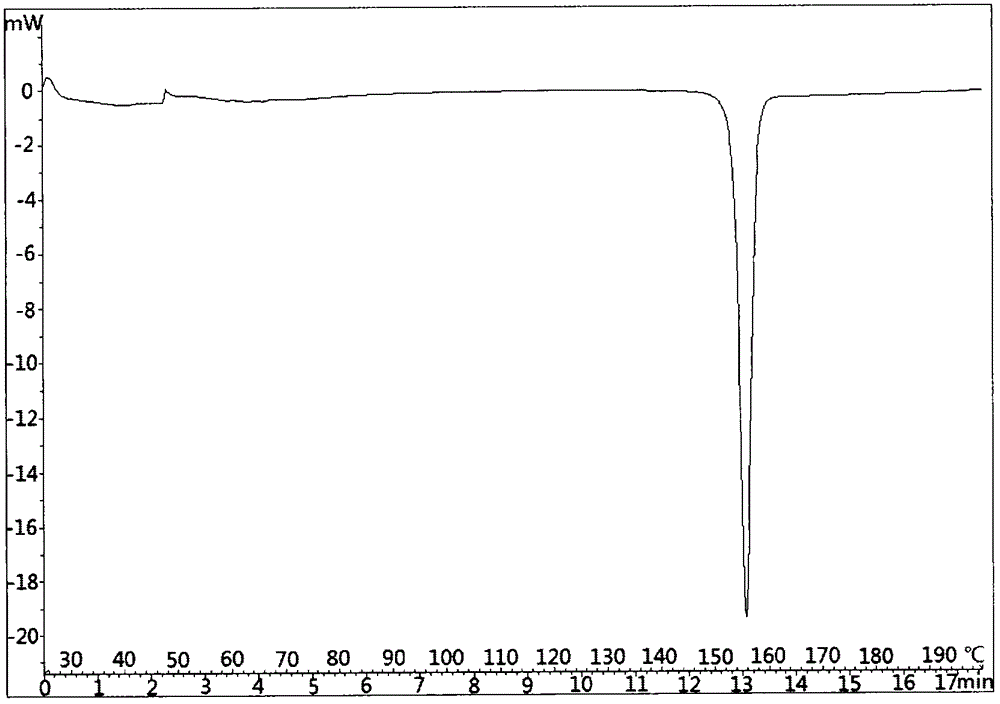
[0140] DSC spectrum such as figure 2 shown. Display: the melting point is 154.8°C.
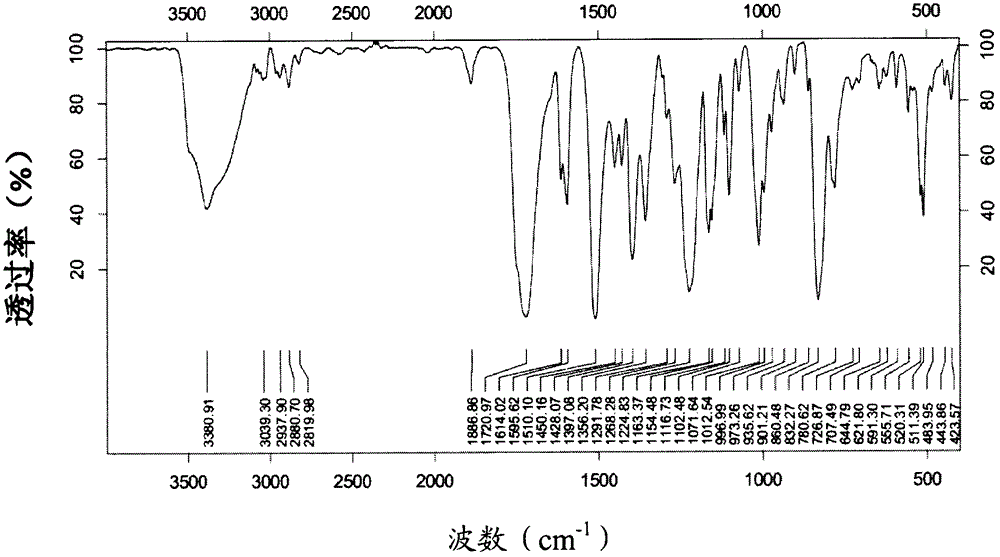
[0141] Infrared spectrum such as image 3 shown.
Embodiment 3
[0142] Embodiment 3: the preparation of crystal form I
[0143] The operation process is the same as in Example 2, except that methanol is replaced by tetrahydrofuran, isopropanol, acetone, n-propanol, acetic acid, n-butanol, ethyl acetate, acetonitrile and N, N-dimethylformamide, respectively to obtain The crystal form I. The XRPD spectrum of the obtained crystal form I is consistent with the XRPD spectrum of the crystal form I in Example 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com