Salen-Fe-like complex, and preparation method and application thereof
A kind of complex and type of technology, applied in the direction of iron-group organic compounds without C-metal bonds, organic compound/hydride/coordination complex catalysts, iron-organic compounds, etc., can solve the problem of cost increase, harsh reaction conditions, step cumbersome and other problems, to achieve the effect of convenient operation, easy control of conditions, and good selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
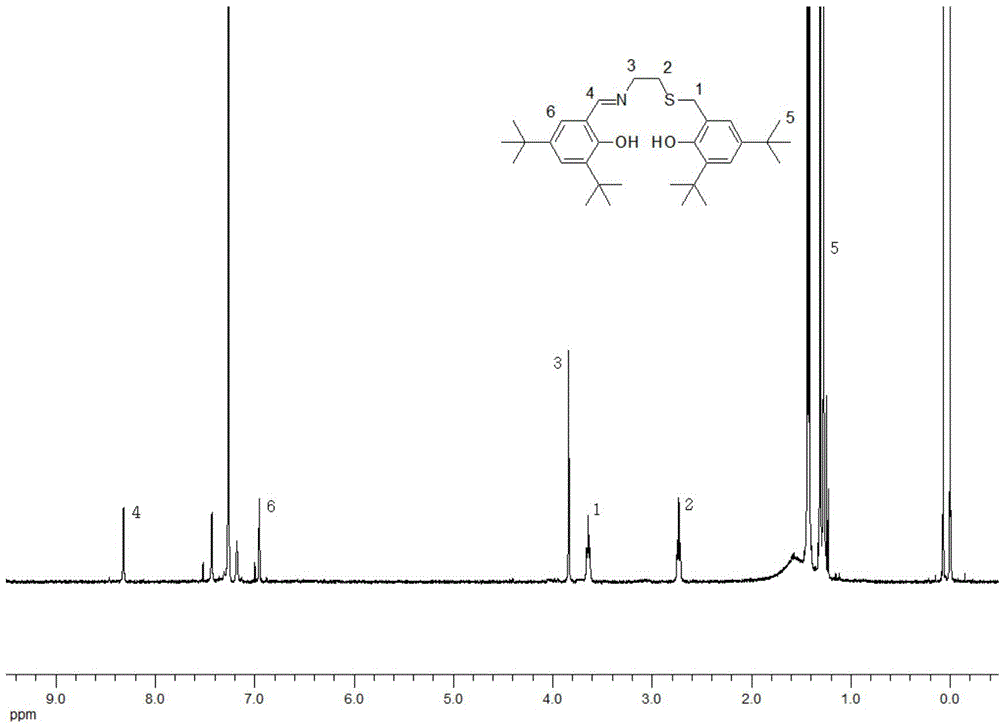
[0032] In the first step, put 0.1mol 2,4-di-tert-butylphenol in a three-necked round-bottomed flask, dissolve in anhydrous methanol (the amount of methanol should be dissolved), add 0.15mol sodium hydroxide, stir at room temperature for 30min, then add 25ml Formaldehyde solution (concentration: 37%-40% (mass fraction)), after adding, stir at room temperature for 26 hours. Pour the resulting light yellow reaction solution into a beaker filled with 250ml of distilled water, the solution becomes turbid and large yellow particles are formed, and the pH is adjusted to between 2 and 3 with concentrated hydrochloric acid (concentration is 37.5%). Extracted with dichloromethane, combined and washed with saturated brine, a small amount several times to obtain a light yellow clear solution. Dry with anhydrous sodium sulfate, filter with suction, and rotary evaporate to obtain a light yellow solid. Recrystallized with petroleum ether and dried to obtain white needle-like crystals, namel...
Embodiment 2
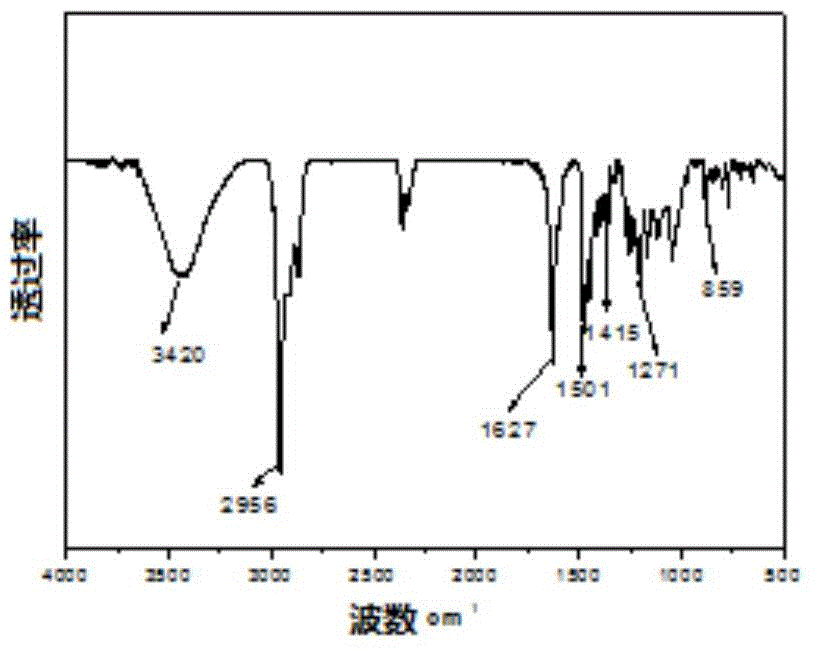
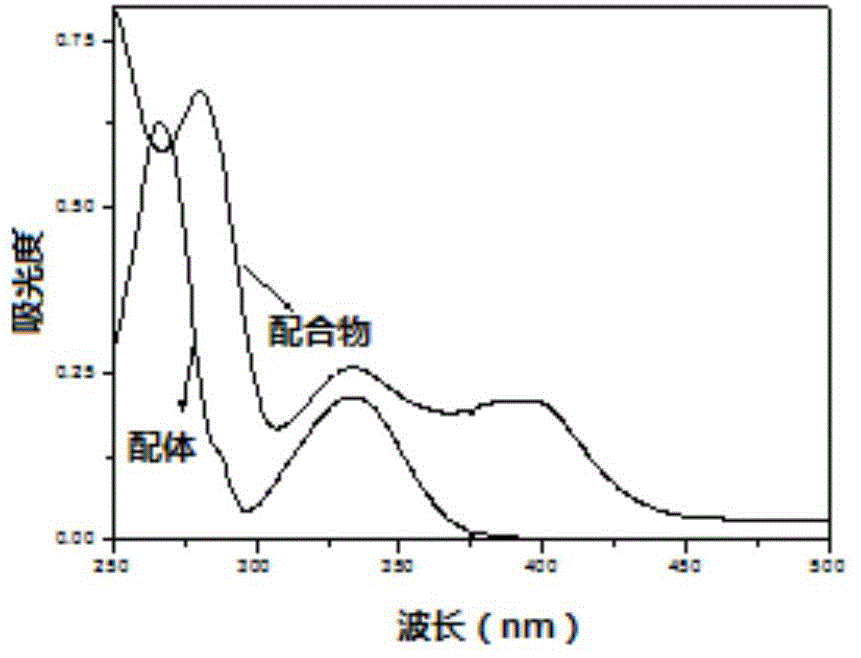
[0036] Take by weighing the class Salen ligand 0.5mmol Lig that obtains in the example 1 1 h 2 Put it in a branch bottle, pump it with argon three times, and under the protection of argon, add dichloromethane to dissolve it, then add dropwise a methanol solution containing 1mmol anhydrous ferric chloride and 1.5mmol anhydrous sodium acetate, Stir at room temperature for 24 hours to obtain a black liquid, which was rotary evaporated, extracted with dichloromethane, washed with saturated brine, and the organic layer was dried with anhydrous sodium sulfate, left to stand for suction filtration, rotary evaporated, and dried in a vacuum oven to obtain a black metallic Shiny Salen-Fe-like complex Cat1 in 63% yield. Infrared (υin cm -1 ): 2959, 1610, 1539, 1458, 1385, 1255, 1180, 1096, 878, 803, 669, 551, 484. Elemental analysis: C 63.90% (63.94%), H 7.92%, (7.88%), O 5.24% (5.32%), N 2.27% (2.34%), S 5.28 (5.33%), Cl 5.83% (5.90%) . The UV spectrum of the complex Cat1 is attach...
Embodiment 3
[0038] Put 0.5 μmol of bistriphenylphosphine ammonium chloride (PPNCl) into a three-necked round-bottomed flask, pump three times with argon, and then add 0.0125mol of carbon disulfide and 0.025mol of epoxy cyclohexane in sequence, that is, carbon disulfide and epoxy ring The molar ratio of hexane is 1:2. React for 4 hours at 120°C, pour the resulting reaction liquid into a beaker filled with methanol to obtain a precipitate, filter with suction to obtain a precipitate, wash with methanol, and dry in vacuo to obtain 0.13 g of the product . 1 HNMR: (400MHz, CDCl 3 ,δin ppm): 1.43~1.52(m,2H,CH 2 ),1.67~1.77(m,2H,CH 2 ),1.91~2.00(m,2H,CH 2 ),2.20~2.23(m,2H,CH 2 )), 4.05 ~ 4.13 (m, H, CHS), the product's infrared spectrum, nuclear magnetic hydrogen spectrum, nuclear magnetic carbon spectrum, respectively see attached Figure 4 , 5 , 6, these characterizations indicated that the product was a cyclic trithiocarbonate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com