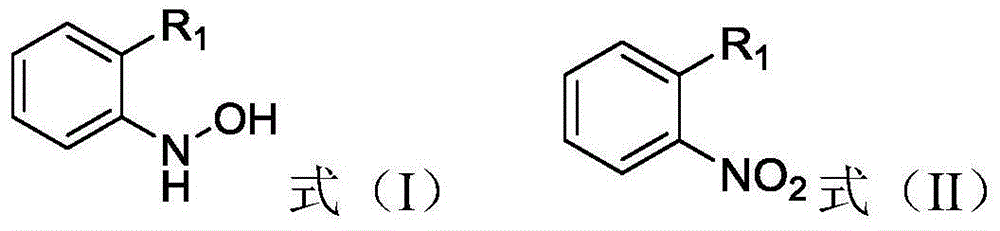Preparation methods of aromatic hydroxylamine compound and N-aromatic acylated hydroxylamine compound
A technology for aromatic hydroxylamines and compounds, which is applied in the field of preparation of N-acylated aromatic hydroxylamine compounds, can solve the problems of poor selectivity, unfavorable industrial production, and low selectivity of reduction products, so as to improve conversion rate and selectivity, avoid The effect of reaction product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
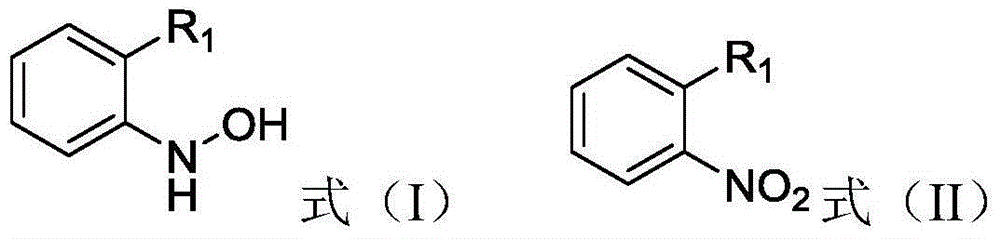
[0017] The present invention provides a method for preparing an aromatic hydroxylamine compound represented by general formula (I), the method comprising: in the presence of a reduction catalyst, the compound represented by general formula (II) and hydrazine hydrate in an organic solvent Carry out reduction reaction, described organic solvent is at least one in aliphatic ether and cyclic ether,
[0018]
[0019] Among them, R 1 is hydrogen, halogen, cyano, C 1 -C 4 Alkyl, C 1 -C 4 Haloalkyl or -CH 2 -O-R 2 , where R 2 is substituted or unsubstituted phenyl, substituted or unsubstituted naphthyl, substituted or unsubstituted pyridyl, substituted or unsubstituted pyrazinyl, substituted or unsubstituted pyrimidinyl, substituted or unsubstituted pyridazinyl , substituted or unsubstituted pyrazolyl or substituted or unsubstituted imidazolyl.
[0020] for R 1 Group, halogen can be fluorine, chlorine, bromine or iodine, C 1 -C 4 The alkyl group can be methyl, ethyl, pro...
Embodiment 1
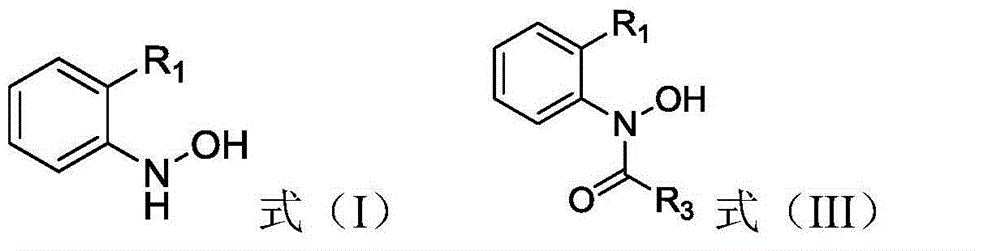
[0044] This embodiment is used to illustrate compound N-[2-[[1-(4-chlorophenyl)pyrazol-3-yl]oxymethyl]phenyl]hydroxylamine shown in formula (Ia) and formula (IIIa) The preparation method of the compound N-hydroxyl-N-(2-(N'-(4-chlorophenyl)pyrazol-3'-yloxymethyl)phenyl)carbamate is shown.
[0045]
[0046] In a four-neck flask equipped with mechanical stirring, a thermometer, and a condenser tube, add 16.5g (50mmol) 2-[(N-4-chlorophenyl)-1H-pyrazole-3-oxymethyl]nitrobenzene, 0.2g Raney nickel and 30mL methyl tert-butyl ether, stir to make it fully mixed, add 50mmol of hydrazine hydrate with a concentration of 80% dropwise to the mixture at 30°C, and react at 30°C for 10 hours after the addition is completed. The target product was obtained, and the conversion rate of the reaction was measured to be 99%, and the selectivity was 96%.
[0047] Then, 4.7g (50mmol) methyl chloroformate was added dropwise to the above-mentioned reduction product, and after the dropwise addition, ...
Embodiment 2
[0049] This example is used to illustrate the preparation method of the compound o-methylphenyl hydroxylamine shown in formula (Ib) and the compound N-hydroxy-N-(2-methylphenyl)carbamate shown in formula (IIIb).
[0050]
[0051] In a four-neck flask equipped with a mechanical stirrer, a thermometer, and a condenser, add 6.9 g (50 mmol) of 2-nitrotoluene, 0.7 g of Raney cobalt, and 120 mL of tetrahydrofuran, stir to make them fully mixed, and pour 150 mmol of hydrazine hydrate with a concentration of 80% was added dropwise to the mixture, and the mixture was reacted at -10° C. for 2 hours to obtain the target product. The conversion rate of the reaction was measured to be 99%, and the selectivity was 95%.
[0052] Then add 4.7g (50mmol) methyl chloroformate dropwise in the above-mentioned reduction product, dropwise add and react at-10 ℃ for 2 hours, obtain the target product, record the conversion rate of reaction to be 99%, selectivity is 96% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com