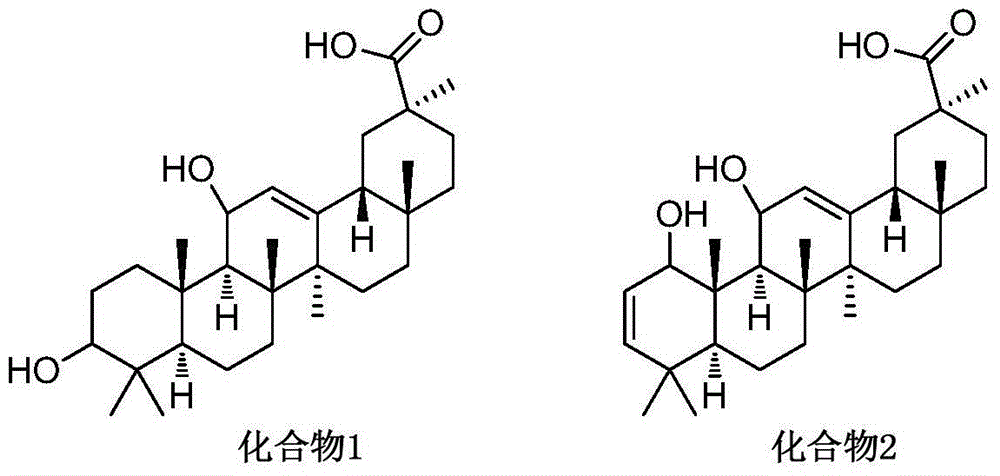
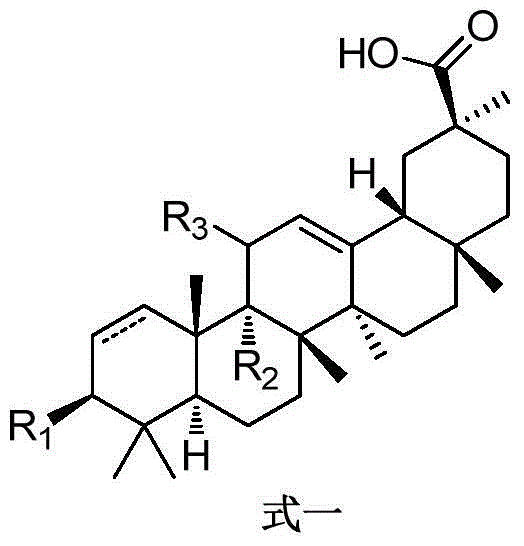
GAOH derivative and medical application thereof
A derivative, C3-C6 technology, applied in the field of medicine, can solve problems such as insignificant effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
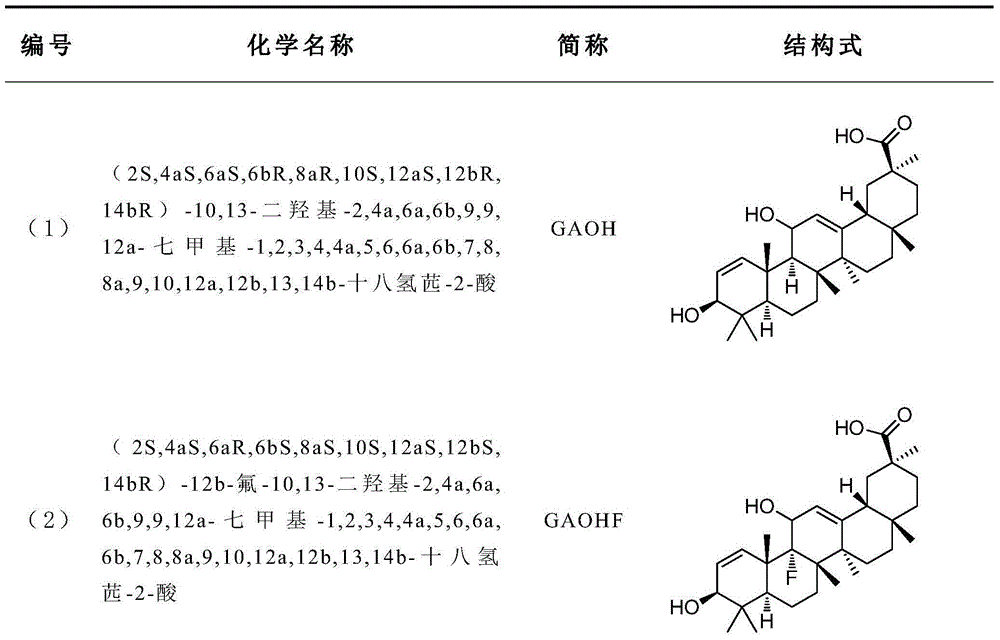
[0036] (2S,4aS,6aS,6bR,8aR,10S,12aS,12bR,14bR)-10,13-dihydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-1,2,3 , Preparation of 4,4a,5,6,6a,6b,7,8,8a,9,10,12a,12b,13,14b-octahydroperylene-2-acid (GAOH for short)
[0037]
[0038] The preparation of formula n compound
[0039]Dissolve 4.7g of the compound of formula m in 100mL of a mixture of acetone and water with a volume ratio of 1:1.2, stir for 15min, control the temperature at 5°C, add 10mL of Jones reagent dropwise, after the dropwise addition, turn to room temperature and continue stirring , TLC detects that the reaction is complete, filters, collects the filtrate, reclaims acetone, the residue is neutralized to neutral with saturated anhydrous sodium bicarbonate, filters, extracts three times with 300mL dichloromethane, combines the dichloromethane layers, concentrates, and dries to prepare Obtain formula n compound;
[0040] The preparation of formula p compound
[0041] Get 0.468g formula n compound, 0.22g SeO 2 In a 50mL...
Embodiment 2
[0046] (2S,4aS,6aR,6bS,8aS,10S,12aS,12bS,14bR)-12b-fluoro-10,13-dihydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-1 , Preparation of 2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,12a,12b,13,14b-octahydroperylene-2-acid (GAOHF for short):
[0047]
[0048] Under nitrogen protection, 4.86g formula a compound and 4.09gCeCl 3 ·7H 2 Dissolve O in 150mL methanol, cool down to -20°C, and quickly add 0.41g NaBH 4 , TLC detected that the reaction was complete; the solvent was removed by rotary evaporation, dissolved in 150 mL of ethyl acetate, washed successively with 10% hydrochloric acid aqueous solution and saturated brine, dried over anhydrous sodium sulfate, filtered, and ethyl acetate was removed by rotary evaporation to obtain GAOHF.
Embodiment 3
[0050] (2S,4aS,6aR,6bS,8aS,10S,12aS,12bS,14bR)-12b-fluoro-10,13-dihydroxy-2,4a,6a,6b,9,9,12a-heptamethyl-1 ,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-eicosan-2-acid (referred to as GAOHF -1) Preparation
[0051]
[0052] The preparation of formula c compound
[0053] Under nitrogen protection, dissolve 4.7g of the compound of formula b in 150mL of methanol, cool down to -20°C, and quickly add 0.41g of NaBH 4 , TLC detected that the reaction was complete; the solvent was removed by rotary evaporation, dissolved in 150 mL of ethyl acetate, washed successively with 10% aqueous hydrochloric acid and saturated brine, dried over anhydrous sodium sulfate, filtered, and ethyl acetate was removed by rotary evaporation to obtain the compound of formula c .
[0054] The preparation of formula d compound
[0055] Add 4.72g of the compound of formula c into a 250mL round bottom flask, add 100mL of DMF and 2.4mL of Py, stir, and slowly drop into 3mL of CH at 0°C 3 SO 2 Af...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com