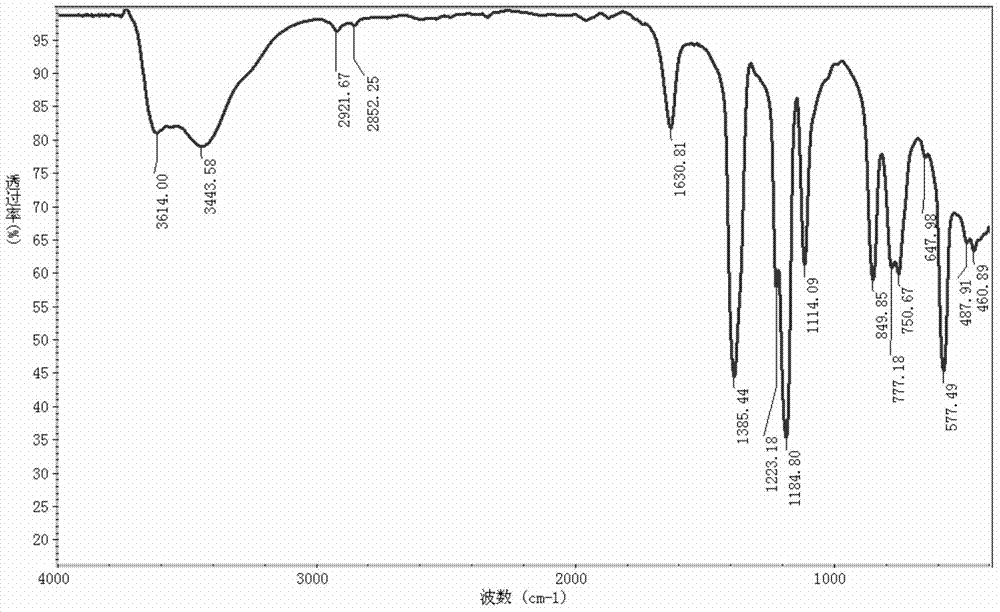
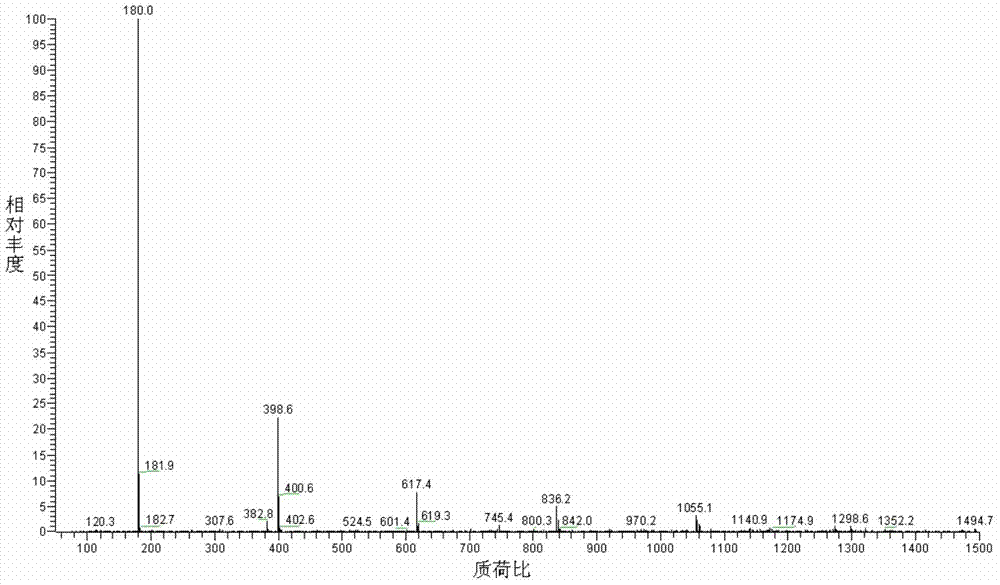
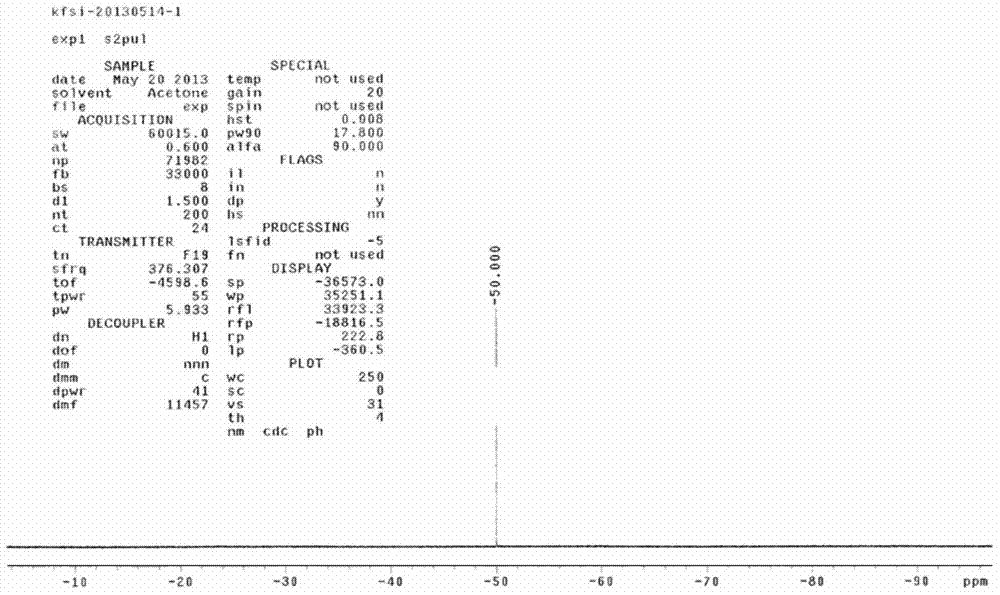
Preparation method of lithium bis(fluorosulfonyl)amide
A technology of lithium bisfluorosulfonyl imide and bisfluorosulfonyl imide, which is applied in the field of preparation of lithium bisfluorosulfonyl imide, can solve problems such as high price, affecting product application performance, and high price of fluorosulfonic acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] The invention provides a kind of preparation method of lithium bisfluorosulfonyl imide, comprising the following steps:
[0034] A) sulfuryl chloride and ammonia are reacted in the presence of an organic base to obtain an organic base salt of dichlorosulfonimide;
[0035] B) mixing the organic base salt of bischlorosulfonimide obtained in step A) with HF, and performing a fluorination reaction to obtain the organic base salt of bisfluorosulfonimide;
[0036] C) mixing the organic alkali salt of bisfluorosulfonimide obtained in step B) with an alkaline substance, and performing a neutralization reaction to obtain a crude alkali metal salt of bisfluorosulfonimide;
[0037] D) purifying the crude bisfluorosulfonimide alkali metal salt obtained in the step C) to obtain the bisfluorosulfonimide alkali metal salt;
[0038] E) mixing the alkali metal bisfluorosulfonyl imide obtained in the step D) with a lithiation reagent, and performing a displacement reaction to obtain lithi...
Embodiment 1
[0092] Under nitrogen protection conditions, 200g of ethyl acetate and 166.7g (1.65mol) of triethylamine were added to a 1L reactor, then the reactor was cooled to 5°C, and then 135g (1.0mol) of sulfuryl chloride was added to the reactor , and then slowly introduce 8.5 g (0.5 mol) of anhydrous ammonia gas into the reactor within 1 h under the control of the flow meter. After the introduction of ammonia gas is completed, the reaction is continued at 5° C. for 0.5 h. Then the cooling was stopped, and the reaction solution was allowed to warm up to room temperature naturally, and the reaction was continued for 48 h. After the reaction, the reaction solution was filtered to remove the triethylamine hydrochloride generated during the reaction to obtain the ethyl acetate filtrate of dichlorosulfonimide triethylamine salt.
[0093] Under the protection of nitrogen, the ethyl acetate filtrate of the above dichlorosulfonimide triethylamine salt was added to a 1L reaction kettle, cooled...
Embodiment 2
[0106] Under nitrogen protection, 200g ethyl acetate and 130.35g (1.65mol) pyridine were added to a 1L reactor, then the reactor was cooled to 5°C, then 135g (1.0mol) of sulfuryl chloride was added to the reactor, and then Under the control of the flow meter, 8.5 g (0.5 mol) of anhydrous ammonia gas was slowly introduced into the reactor within 1 hour. After the introduction of the ammonia gas was completed, the reaction was continued at 5° C. for 0.5 hour. Then stop cooling, allow the reaction solution to naturally warm up to room temperature, and continue the reaction for 48 hours. After the reaction, the reaction solution is filtered to filter out the pyridine hydrochloride generated during the reaction to obtain ethyl acetate of bischlorosulfonimide pyridine salt. ester filtrate.
[0107] Under nitrogen conditions, the ethyl acetate filtrate of the obtained bischlorosulfonimide pyridinium salt was added to a 1L reactor, cooled to 0°C, and 30g of HF was introduced therein u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com