Method for polypeptide biomimetic preparation of platinum catalyst for fuel battery and application of platinum catalyst for fuel battery
A catalyst, platinum nanotechnology, used in chemical instruments and methods, physical/chemical process catalysts, battery electrodes, etc., can solve problems such as voltage and current instability, affect performance, and uneven nanomaterials, and achieve mild and good conditions. Anti-agglomeration, strong tunable denaturation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
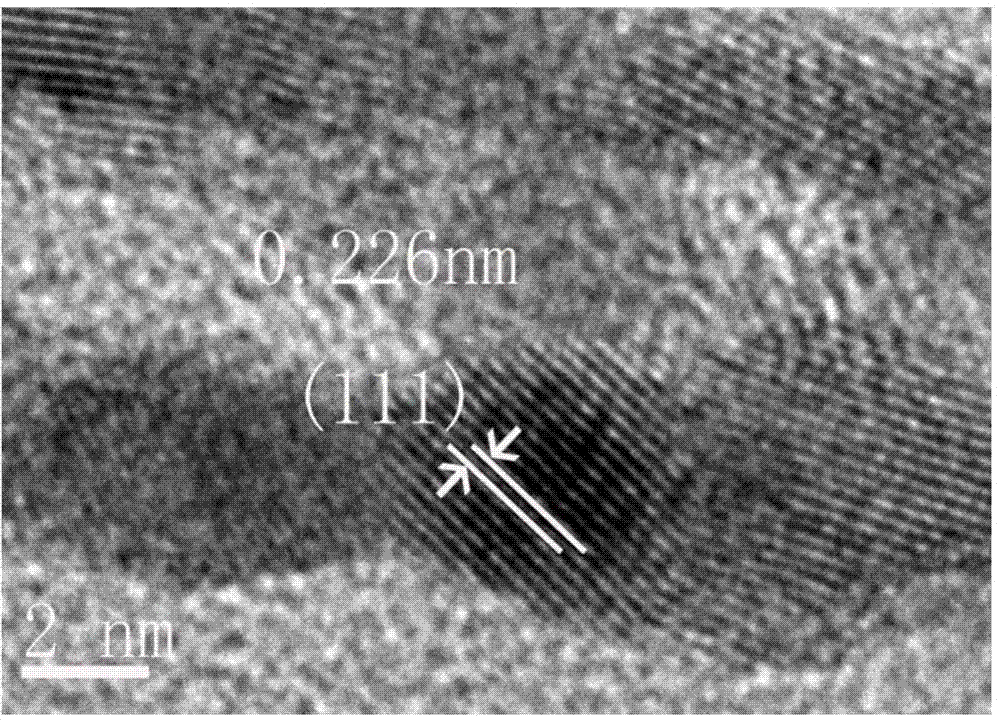
Image
Examples
Embodiment 1
[0075] Peptide I 3 K can self-assemble into a one-dimensional nanofiber structure with a diameter of about 10 nm and a length of several microns at room temperature, and this fiber can be used as a template for platinum nanofibers. At room temperature, the I 3 K aqueous solution and K 2 PtCl 4 Aqueous solutions are mixed, where K 2 PtCl 4 The aqueous solution concentration is 1mM, I 3 The concentration of K is 0.5mM, 0.2mM, 0.1mM respectively, and it is formulated into I 3 K and K 2 PtCl 4 Mixed solutions with concentration ratios of 1:2, 1:5, and 1:10, after mixing evenly, add 1mM NaBH 4 The aqueous solution was reduced, and the one-dimensional platinum nanofiber suspension reacted for 0.5 hours was centrifuged at 10,000 rpm for 7 minutes, then repeatedly added with water for at least 3 times, and then ultrasonically dispersed in the aqueous solution again.
[0076] The prepared one-dimensional platinum nanofiber dispersion was drop-coated on a clean glassy carbon el...
Embodiment 2
[0085] Peptide I 3 H, I 3 CGK, I 4 K 2 1. Fmoc-FFGCK can self-assemble into a one-dimensional nanofiber structure with a diameter of about 10 nm and a length of several microns under mild conditions, and the fiber can be used as a template for platinum nanofibers. Will I 3 K aqueous solution and K 2 PtCl 4 Aqueous mixing, K 2 PtCl 4 The concentration of the aqueous solution is 1mM and the concentration of the peptide template is kept constant at 0.1mM, so that the concentration ratio of the peptide template and the platinum precursor is 1:10. After mixing evenly, add 1mM NaBH 4 The aqueous solution was reduced, sealed and shaken for 1 minute, then allowed to stand for 0.5 h, centrifuged and electrochemically characterized (the method was the same as in Example 1). The ECSA of one-dimensional platinum nanofibers prepared by each template is shown in Table 3. It can be seen that the catalytic activity of one-dimensional platinum nanofibers prepared under different templa...
Embodiment 3
[0093] with I 3 K is template, Pt precursor is H 2 PtCl 6 , the concentration ratio of 1:10 remains unchanged, and the peptide solution is mixed with K 2 PtCl 4 Mix the solutions so that the concentration ratio is 1:10. After mixing evenly, reduce them with 1mM sodium borohydride + 1mM ascorbic acid, or 1mM formic acid, or 1mM hydrazine hydrate aqueous solution, and shake them in a closed place for one minute, then place them at 80°C to continue After reacting for 24 hours, perform electrochemical characterization after centrifugation (the method is the same as in Example 1). The ECSA of the one-dimensional platinum nanofibers reduced by each reducing agent is shown in Table 5. It can be seen that the catalytic activity of the one-dimensional platinum nanofibers reduced by each reducing agent is higher than that of the commercial Pt / C catalyst.
[0094] Table 5. ECSA of 1D Pt nanofibers prepared with different reducing agents
[0095]
[0096] The electrocatalytic expe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com