Quality inspection method of anti-hysteritis soft capsules
A quality inspection method and soft capsule technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of incomplete quality control indicators and inability to control product quality, so as to achieve simple and easy quality inspection methods and ensure clinical efficacy , high precision effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example
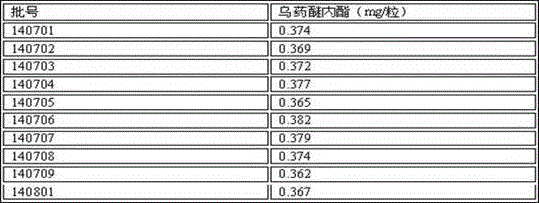
[0025] Experimental example: Determination of the content of hiraegina lactone
[0026] (1) Selection of mobile phase:
[0027] Mobile phase 1: methanol-acetonitrile-water (35:25:40);
[0028] Mobile phase 2: methanol-acetonitrile-water (355:255:40);
[0029] Mobile phase 3: methanol-water (60:40);
[0030] Mobile phase 4: methanol-water (40:60);
[0031] Mobile phase 5: acetonitrile-water (60:40);
[0032] Mobile phase 6: acetonitrile-water (56:44);
[0033] Through the analysis of the above comparative test results, mobile phase 6 has the characteristics of appropriate retention time, good sample separation effect, negative and no interference, etc. At the same time, according to the analysis and conclusion of follow-up experiments, when the number of theoretical plates of huigui lactone reaches 3000, the separation is greater than 1.5, so the number of theoretical plates based on the peak of huigui lactone should not be less than 3000.
[0034] (2) Screening of ...
Embodiment
[0061] A quality detection method for Kanggongyan Soft Capsules, comprising the following steps:
[0062] (1) Identification of Guangdong Zizhu:
[0063] Take 2 g of the content of this product, add 50ml of 60% ethanol, 1ml of hydrochloric acid, heat and reflux on a water bath for 4 hours, filter, steam the filtrate on a water bath to 15ml, add 20ml of water, shake well, filter, and shake the filtrate with ether Extract 3 times, 15ml each time, combine the ether solution, put it on a water bath and evaporate to dryness, add 1ml methanol to the residue to dissolve, and use it as the test solution. Another 5 g of Guangdong Zizhu reference medicinal material was taken, and the reference medicinal material solution was prepared in the same way. According to the test of thin-layer chromatography (Appendix VI B, Chinese Pharmacopoeia 2010 Edition), draw 5 μl of each of the above two solutions, spot them on the same silica gel G thin-layer plate, and mix with toluene-ethyl formate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com