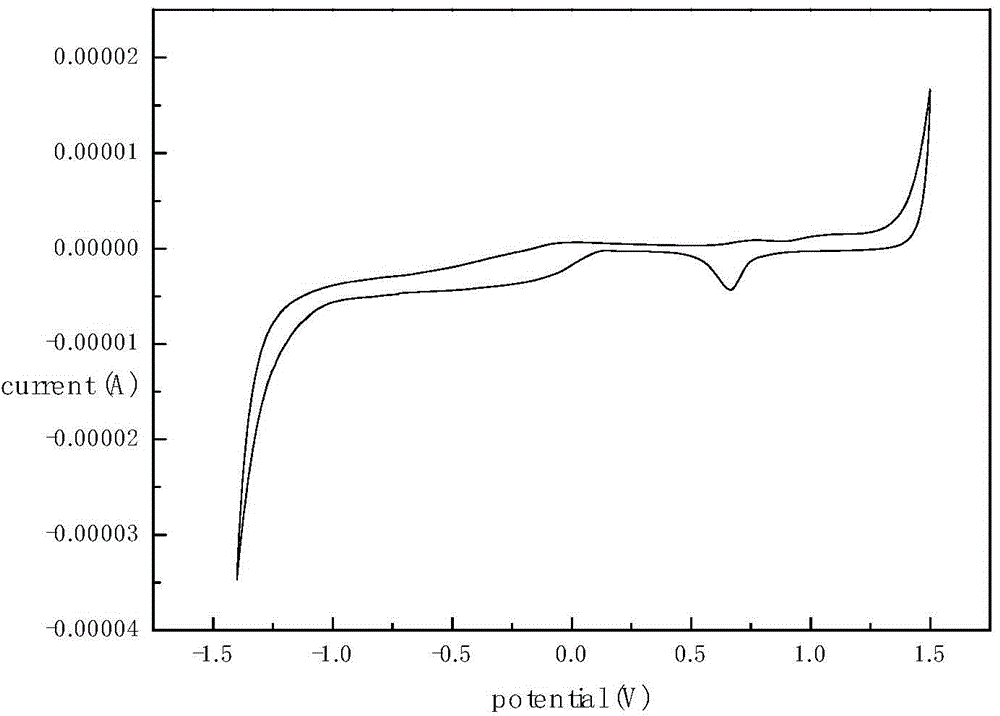
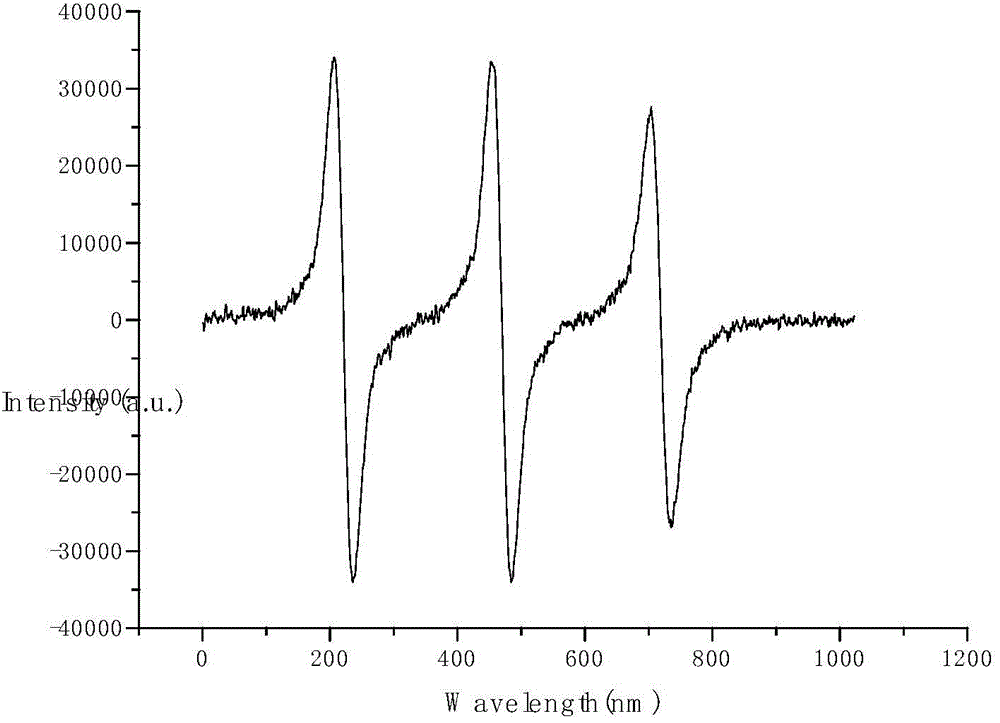
CT-(MIO) modified polyphosphazene as well as synthesis method and application thereof
A technology of indene nitrogen oxide and free radicals, which is applied in the field of isoazaindene nitrogen oxide radical modification polyphosphazene and its synthesis, which can solve the problems of difficult energy density and cycle stability, poor solubility and processability, and charge-discharge ratio Low capacity and other issues, to achieve the effect of good dissolution and processing performance, high power density, high charge storage density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Poly(diisozaindene nitroxide radical aminophosphazene)
[0046] In a single-necked flask of 250 mL of sulfamic acid (0.1165 g, 1.2 mmol), calcium sulfate dihydrate (0.1033 g, 0.6 mmol), ammonium chloride (20.0 g, 0.374 mmol), phosphorus pentachloride (66.0 g, 0.317 mmol) mmol) and 1,2,4-trichlorobenzene (23mL), quickly heated up to 210°C for 0.5h, then cooled to 190°C for 3h, cooled to room temperature after the reaction, filtered, reprecipitated with petroleum ether, and then used Tetrahydrofuran was dissolved in petroleum ether to reprecipitate, and the oil pump was vacuum-dried at room temperature to obtain polydichlorophosphazene.
[0047] Under nitrogen protection, polydichlorophosphazene (0.058g, 1mmol), tetrahydrofuran (10mL), triethylamine (0.193mL) were added dropwise with 5-amino-1,1,3,3-tetramethyliso A tetrahydrofuran (3ml) solution of azaindene nitroxide radical (0.218, 1mmol) was added dropwise and reacted at room temperature for 12 hours. After the react...
Embodiment 2
[0051] Poly(isozaindene nitroxide radical amine-anilino-phosphazene)
[0052] In a single-necked flask of 250 mL of sulfamic acid (0.1165 g, 1.2 mmol), calcium sulfate dihydrate (0.1033 g, 0.6 mmol), ammonium chloride (20.0 g, 0.374 mmol), phosphorus pentachloride (66.0 g, 0.317 mmol) mmol) and 1,2,4-trichlorobenzene (23mL), quickly heated up to 210°C for 0.5h, then cooled to 190°C for 3h, cooled to room temperature after the reaction, filtered, reprecipitated with petroleum ether, and then used Tetrahydrofuran was dissolved in petroleum ether to reprecipitate, and the oil pump was vacuum-dried at room temperature to obtain polydichlorophosphazene.
[0053] Under nitrogen protection, polydichlorophosphazene (0.058g, 1mmol), tetrahydrofuran (10mL), triethylamine (0.193mL) were added dropwise with 5-amino-1,1,3,3-tetramethyliso Azaindene nitrogen oxide radical (0.109, 0.5mmol) and aniline (0.047g, 0.5mmol) in tetrahydrofuran (3mL) solution, after the dropwise addition, react at...
Embodiment 3
[0055] Poly(diisozaindene nitroxide radical vinyl phosphazene)
[0056] Put 3g of hexachlorocyclotriphosphazene monomer into a clean polymerization tube that has been dried with an alcohol blowtorch. After the polymerization tube is connected to a vacuum and inert gas device, turn on the pump to pump for 30 minutes, and then pass in nitrogen to pump. vacuum. After repeating three to four times, close the valve. Melt the hexachlorocyclotriphosphazene in the polymerization tube, cool it down to room temperature, re-evacuate for about 3 hours, and then seal the tube with an alcohol torch. Put the sealed polymerization tube into an oil bath at 250°C for polymerization. During the polymerization, observe the situation in the polymerization tube at any time. Take the polymerization tube out of the oil bath, put it in a glove box filled with nitrogen, and break the polymerization tube. The polymer was taken out from the tube, shredded and put into a round bottom flask, and dry pet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com