Synthesis technology of cefoxitin acid
A technology for cefoxitin acid and synthesis process, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of high cost, difficult to obtain cephalosporanic acid, low yield and the like, achieves reduction of energy consumption and discharge of organic waste water, and is suitable for large-scale industrialization Production, yield and product quality effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
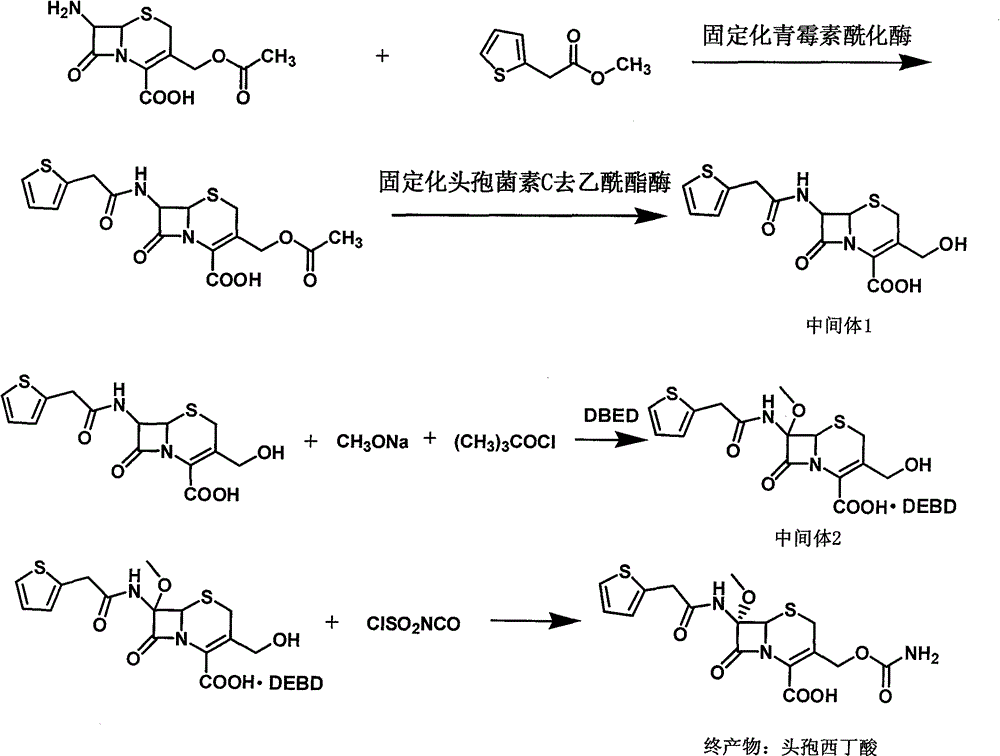
[0043] The synthesis method of cefoxitin acid of the present invention includes the following steps:
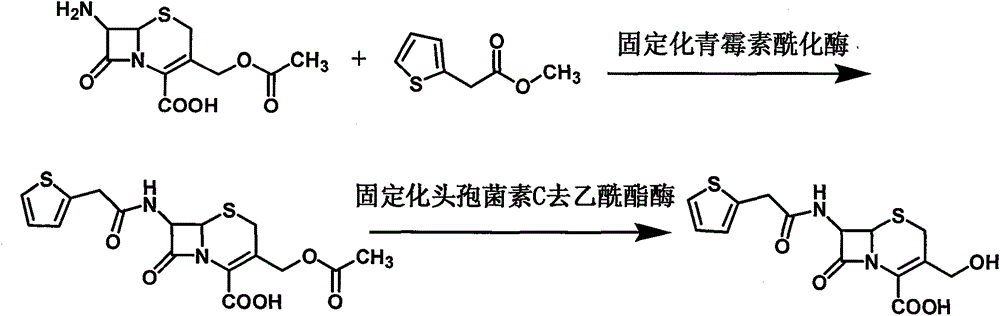
[0044] 1) Dissolve 7-ACA in water, adjust the pH to 7-ACA with ammonia, then add immobilized penicillin acylase, add 2-thiophene acetate dropwise, filter the filtrate and add immobilized after the reaction is complete The cephalosporin C deacetyl esterase continues to react. After the reaction, the filtrate is filtered out, activated carbon is added for decolorization and then filtered, then the pH is adjusted to crystallize, filtered, and dried to obtain the intermediate 3-deacetylcephalothin acid.
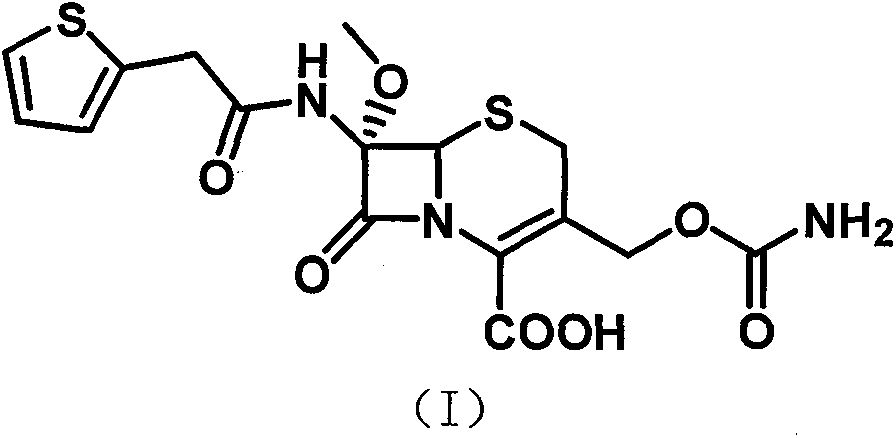
[0045] 2) Dissolve the intermediate 3-deacetyl cefalotinic acid obtained in step 1) with a mixed solvent of dichloromethane, methanol and tetrahydrofuran, drop the sodium methoxide solution prepared in advance and cool down after dropping to a certain temperature. Then add tert-butyl hypochlorite dropwise, then keep the temperature for reaction, hydrolyze after the reaction, adjust t...
Embodiment 1
[0047] Example 1. Synthesis of 3-Deacetyl Cefalotinic Acid
[0048] Put 80 grams of 7-ACA into a flask with stirring, add 1000 ml of deionized water, turn on the stirring and adjust the pH to 7-ACA with 8% ammonia water, control the temperature at 20°C, and put 30 grams of immobilized penicillin for acylation Enzyme, then add 55 grams of methyl 2-thiophene acetate dropwise, while using 8% ammonia to control the pH from 6.5 to 7.0 until the pH does not change. Liquid chromatography detects that the residual 7-ACA is less than 1.0%. The reaction is over, and the filtrate is collected by filtration. Wash the immobilized enzyme with deionized water; add the filtrate to a stirred flask, control the temperature at 20°C, add 20 grams of immobilized cephalosporin C deacetylesterase to start the reaction, and use 8% ammonia to control the pH from 7.0 to 7.5 to pH Keep it for 3 minutes and the reaction is over. Collect the filtrate by filtration. Add 5 g of activated carbon to the filtrate...
Embodiment 2
[0049] Example 2. Synthesis of 7-α-methoxy-3-deacetylcephalothin acid benzathine salt
[0050] Put 100 g of 3-deacetyl cephalotinic acid, 1000 ml of dichloromethane, 100 ml of methanol, and 100 ml of tetrahydrofuran into a stirred flask, stir to dissolve, cool to -85°C with liquid nitrogen, then add dropwise to prepare in advance Sodium methoxide solution (90g sodium methoxide+200ml dichloromethane+200ml methanol), control the temperature -83~-87℃, add 35g tert-butyl hypochlorite dropwise after dropping, continue the reaction after dropping 1 Hour, add 100 ml of 50% glacial acetic acid solution, stir for 15 minutes and increase the temperature to -50°C, then add 300 ml of 5% sodium chloride solution, increase the temperature to 0~5°C, stir for 10 minutes, then stand for separation, water phase Extract once with 100 ml of dichloromethane, combine the organic phases, add activated carbon for decolorization, filter, add 500 ml of water to the filtrate, stir, adjust the pH 7.2 to 7.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com