Method for preparing Invokana key intermediate
An intermediate and key technology, which is applied in the field of key chiral intermediates of canagliflozin for the treatment of diabetes, can solve problems such as the source cost of key intermediates of canagliflozin, and achieve the effect of solving preparation and cost problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The preparation method of the present invention is further described in detail below by way of examples.
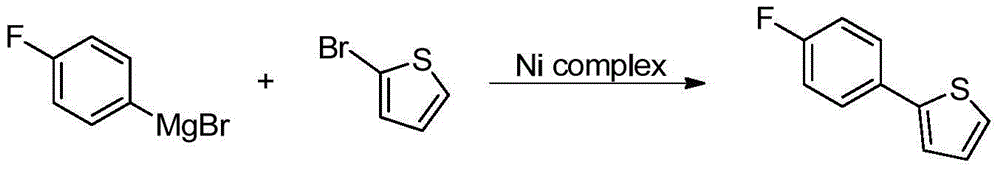
[0014] 1. Preparation of 2-(4-fluorophenyl)thiophene and p-fluorophenylmagnesium bromide
[0015] Reaction formula:
[0016]
[0017] In a 500mL three-necked round-bottomed flask, magnesium chips (1.5g, 61.3mmol) and one grain of iodine were added under nitrogen protection. After the iodine was heated and sublimated, a small amount of tetrahydrofuran and p-fluorobromobenzene were added. After the reaction was initiated, a solution of p-fluorobromobenzene (10.7 g, 61.3 mmol) in tetrahydrofuran (100 mL) was slowly added dropwise. After the reaction was completed, the reaction system 1 was obtained.
[0018]
[0019] In a 500mL round bottom flask, add 2-bromothiophene (10.0g, 61.3mmol), tetrahydrofuran (100mL), NiCl 2 (dppe) (80mg, 1.5mol%), the reaction system 1 was slowly added dropwise at 0-5°C, after the drop was completed, the reaction was stirred overnig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com