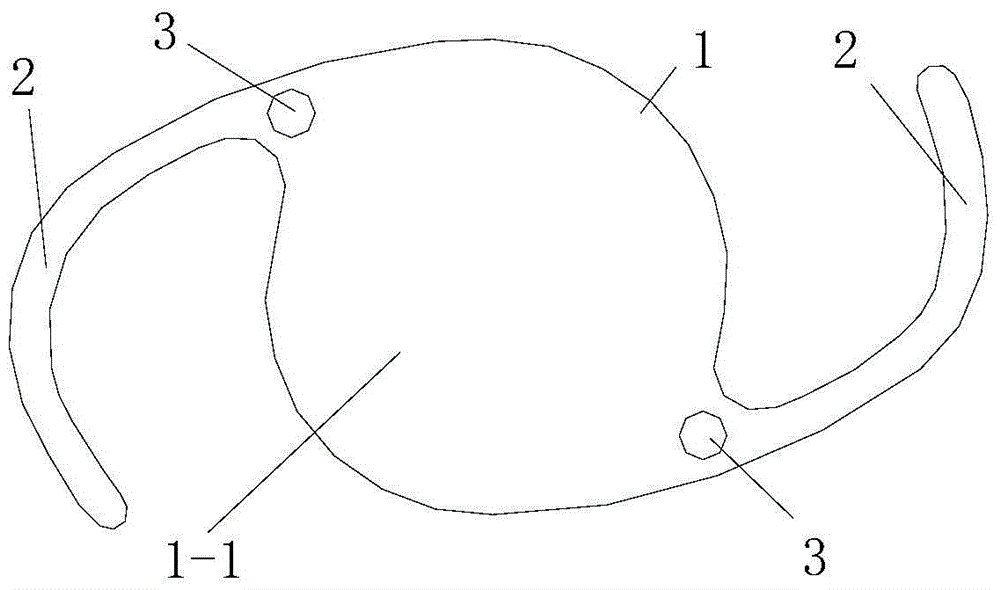
Drug sustained-release microsphere capable of being embedded in intraocular lens loop and preparation method of drug sustained-release microsphere
A technology of intraocular lens and sustained-release microspheres, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-active ingredients, etc., can solve the problem that the sustained release time and concentration of drugs cannot meet the clinical application requirements, and the inhibitory effect and degree of inhibition are not enough. Determine the toxicity and side effects of other tissues in the eye, achieve the effect of simple and effective drug loading and release speed, reduce intraocular inflammation and infection, improve visual quality and long-term vision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The manufacture method of drug sustained-release microspheres comprises the following steps:
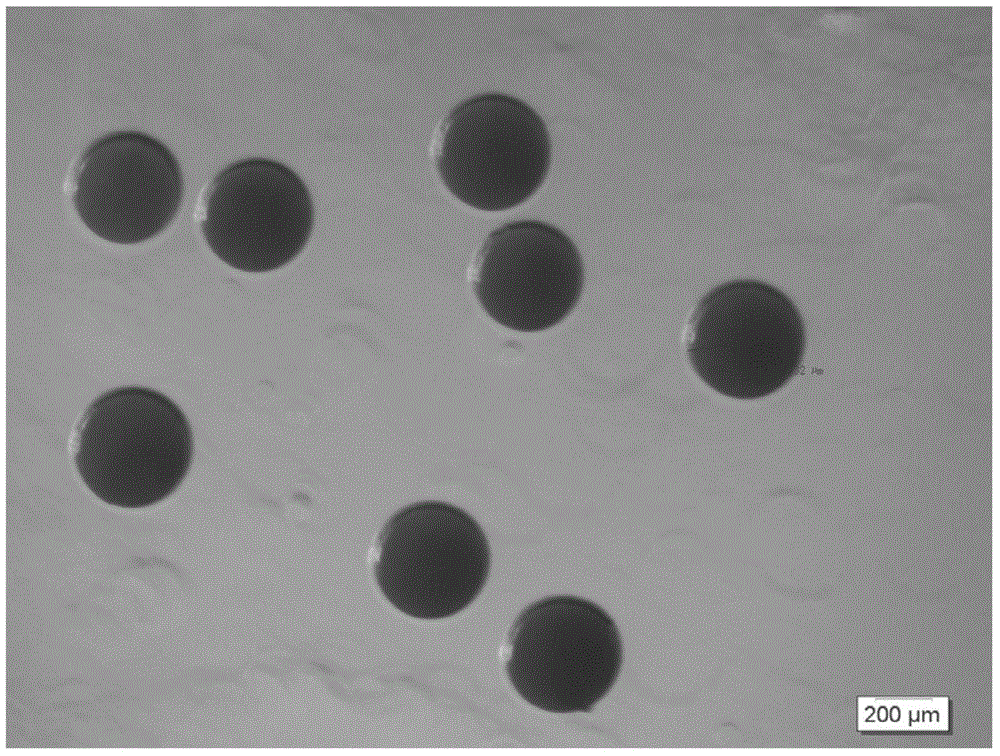
[0040] 1) Put 500mg of gatifloxacin powder and 1500mg of glyceryl tristearate powder in a glass beaker, heat and stir the beaker in a water bath at 80°C until the solid melts; extract 5ml of the above liquid with a syringe and add 1ml / The speed of sec is quickly injected into the nozzle heated to 80°C (the diameter of the nozzle is 1500 microns); at the same time, the 6.8kpa air output from the air compressor passes through the heating nozzle and then sprays out from the nozzle of the nozzle together with the aforementioned liquid (spraying Height of about 1.0m), the above-mentioned liquid is sprayed and condensed into microspheres of 400-800 microns in the air; sterilized by ethylene oxide and packaged in a sealed manner.
example 2
[0042] 1) Put 300mg of fleroxacin powder and 700mg of stearic acid powder in a glass beaker, heat and stir the beaker in a water bath at 75°C until the solid melts; draw 3ml of the above liquid with a syringe and rapidly dissolve it at a rate of 0.5ml / sec Inject into the nozzle heated to 75°C (the diameter of the nozzle is 1000 microns); at the same time, the 7.1kpa air output from the air compressor passes through the heated nozzle and then sprays out from the nozzle of the nozzle together with the aforementioned liquid (the spray height is about 1.5m) ), the above-mentioned liquid is sprayed and condensed into microspheres of 600 microns in the air; sterilized by ethylene oxide, sealed and packaged.
example 3
[0044] 1) Put 400mg of clarithromycin powder, 200mg of oleic acid and 400mg of monoglyceride powder into a glass beaker, heat and stir the beaker in a 70°C water bath until the solid melts; draw 2ml of the above liquid with a syringe and add 1.5ml The speed of / sec is quickly injected into the nozzle heated to 70°C (the diameter of the nozzle is 600 microns); at the same time, the 5.5kpa air output from the air compressor passes through the heated nozzle, and then sprays out from the nozzle of the nozzle together with the aforementioned liquid In a confined space (spraying height of about 2.0m), the above liquid is sprayed and condensed into 200 micron microspheres in the air; sterilized by ethylene oxide and sealed in a package.
[0045] The heating of shower head in above-mentioned 3 embodiments can adopt electric heater or far-infrared heater or alcohol lamp.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com