Preparation method for flucloxacillin sodium
A technology of flucloxacillin sodium and pivaloyl chloride, which is applied in the field of antibiotic drug synthesis, can solve the problems of high product impurities, high product impurities, and low yield, and achieve the effects of good purity, simple operation, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
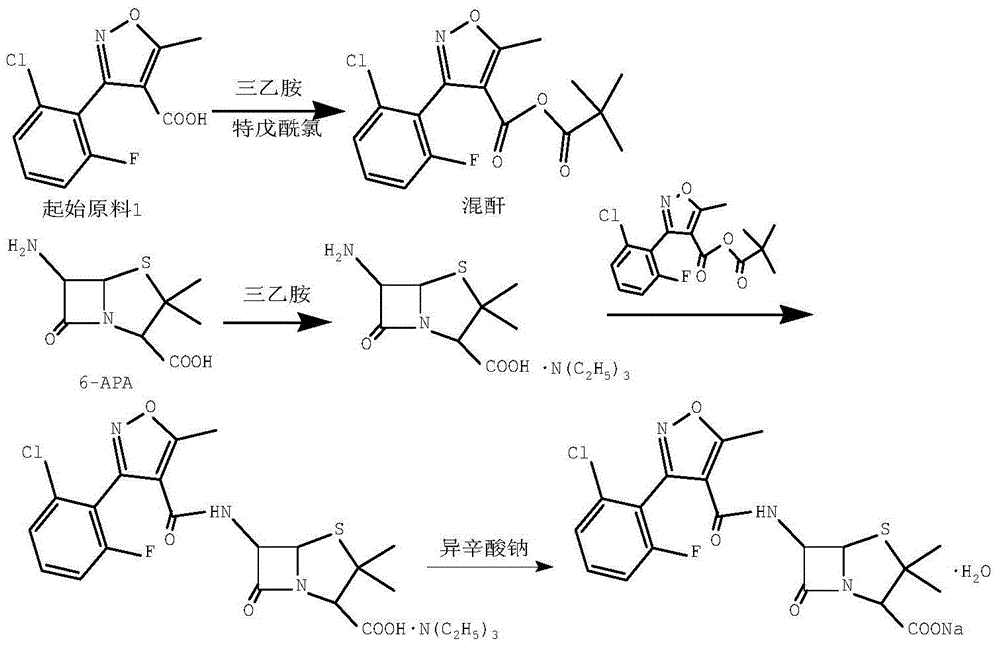
[0027] Add 100ml of dichloromethane and 12.0g (46.9mmol) of starting material 1 into a 250ml three-necked flask, stir, cool down to -5~0°C, add 5.2g (51.6mmol) of triethylamine dropwise, after the material liquid is clarified, drop Add 5.8g (48.3mmol) of pivaloyl chloride, control the temperature at -5-0°C, and react for 40 minutes after dropping to obtain mixed anhydride;
[0028] Add 100ml of dichloromethane, 10.0g (46.2mmol) of 6-APA, 9.3g (92.4mmol) of triethylamine into a 500ml three-neck flask, control the temperature at 30-35°C, and stir until the feed liquid is clear. Lower the temperature to -5°C, add the mixed anhydride prepared in the above steps dropwise, control the temperature at -5-0°C, react for 1 hour, and then distill off the dichloromethane under reduced pressure to obtain an oily substance. Add 30ml acetone in gained oil, stir 10 minutes, remove triethylamine hydrochloride by filtration, obtain flucloxacillin triethylamine salt acetone solution;
[0029] A...
Embodiment 2
[0031] Add 100ml of dichloromethane and 12.0g (46.9mmol) of starting material 1 into a 250ml three-necked flask, stir, cool down to -5~0°C, add 5.2g (51.6mmol) of triethylamine dropwise, after the material liquid is clarified, drop Add 5.8g (48.3mmol) of pivaloyl chloride, control the temperature at -5-0°C, and react for 50 minutes after the dropwise completion to obtain mixed anhydride;
[0032] Add 100ml of dichloromethane, 10.0g (46.2mmol) of 6-APA, 9.3g (92.4mmol) of triethylamine into a 500ml three-neck flask, control the temperature at 30-35°C, stir until the material liquid is clear, then cool down to -5°C, Add the mixed anhydride prepared in the above steps dropwise, control the temperature at 0-5°C, react for 1 h, then distill off the dichloromethane under reduced pressure to obtain an oily substance, add 25ml of acetone to the obtained oily substance, stir for 10 minutes, filter to remove triethylamine Hydrochloride, get flucloxacillin triethylamine salt acetone solu...
Embodiment 3
[0035] Add 100ml of dichloromethane and 12.0g (46.9mmol) of starting material 1 into a 250ml three-necked flask, stir, cool down to -5~0°C, add 5.7g (56.3mmol) of triethylamine dropwise, and after the material liquid is clarified, drop Add 5.9g (49.2mmol) of pivaloyl chloride, control the temperature at -5-0°C, and react for 60 minutes after the dropwise completion to obtain mixed anhydride;
[0036] Add 100ml of dichloromethane, 10.0g (46.2mmol) of 6-APA, 9.3g (92.4mmol) of triethylamine into a 500ml three-neck flask, control the temperature at 30-35°C, stir until the material liquid is clear, then cool down to -5°C, Add the mixed anhydride prepared in the above steps dropwise, control the temperature at -5~0°C, react for 1 h, then distill off the dichloromethane under reduced pressure to obtain an oily substance, add 20ml of acetone to the obtained oily substance, stir for 10 minutes, filter to remove triethyl ether Amine hydrochloride, get flucloxacillin triethylamine salt ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com