Deoxypodophyllotoxin medicine-containing pharmaceutical composition and preparation method and preparation thereof
A technology of deoxypodophyllotoxin and pharmaceutical preparations, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations of non-active ingredients, etc., and can solve problems such as the inability to make glycoside derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
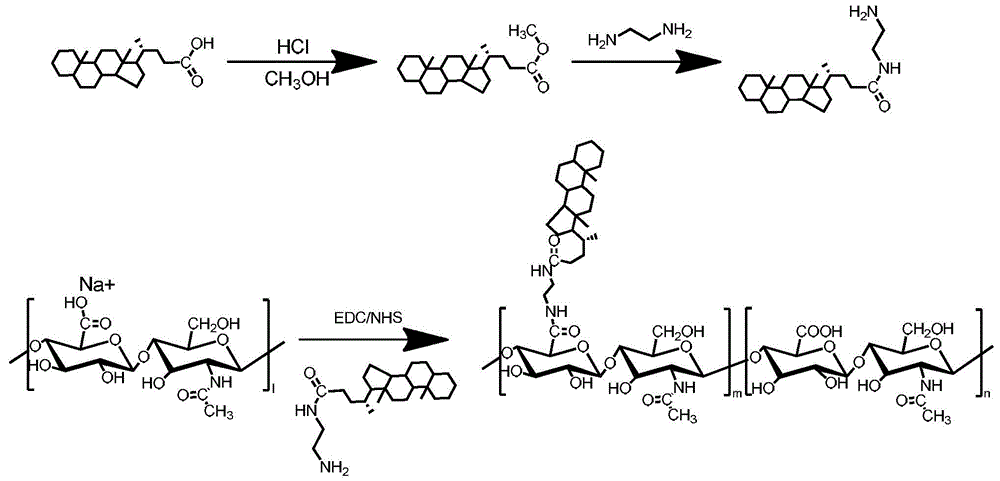
[0091] Preparation method 1 of HA-CA amphiphilic hyaluronic acid copolymer, the reaction equation is as follows figure 1 :
[0092] Sodium hyaluronate was dialyzed against deionized water for 24 hours. After lyophilization, hyaluronic acid (5 mg, 1 mmol carboxyl) was dissolved in 1 mL deionized water.
[0093] Dissolve 10g of 5β-cholanic acid (CA) in 50mL of methanol, add 1.80mL of concentrated hydrochloric acid, seal the container with a film, and react at a constant temperature of 60°C under magnetic stirring for 6 hours, add excess distilled water to form a white precipitate, wash with water 3 times, and dry in vacuum to obtain Methyl cholanoate in white powder form.
[0094] Dissolve 9 g of methyl cholanoate in 50 mL of DMF, slowly add EDA (0.9 μmol), and react with magnetic stirring for 6 hours, then react at a constant temperature of 130°C for 6 hours under magnetic stirring, add excess distilled water to form a white precipitate, wash with water 3 times, and dry in va...
Embodiment 2
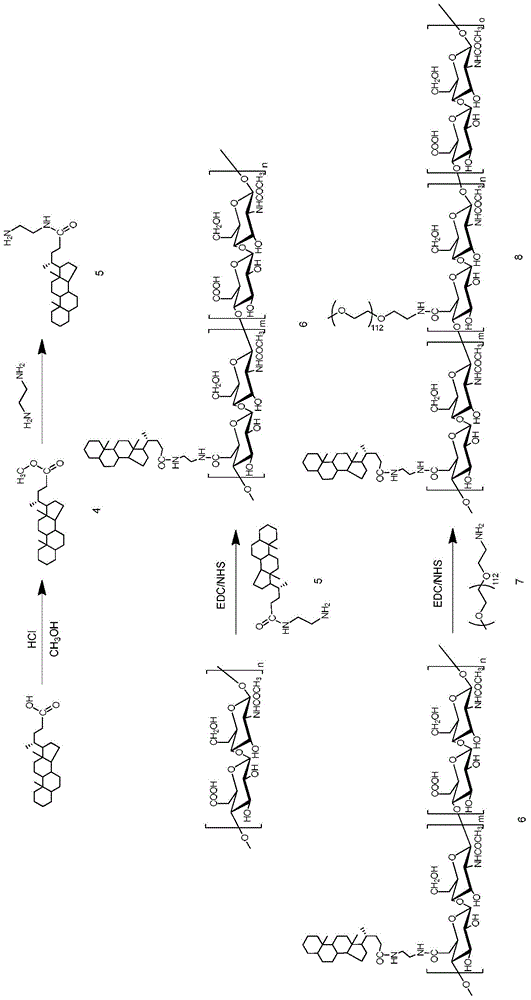
[0098] Preparation method 2 of HA-CA amphiphilic hyaluronic acid copolymer, the reaction equation is as follows figure 2 :
[0099] Sodium hyaluronate was dialyzed against deionized water for 24 hours. After lyophilization, hyaluronic acid (400 mg, 1 mmol carboxyl group) was dissolved in 5 ml deionized water, and 650 μl (1 mmol) tetrabutylammonium hydroxide (TBA) was added and stirred at 60° C. overnight.
[0100] Under the condition of stirring at 60°C, 120 mg of HA-TBA was dissolved in 12 ml of dimethyl sulfoxide (DMSO), and then 5β-cholanic acid (CA, 39.8 mg, 98.8 μmol),) EDC (72.8 mg, 379.8 μmol) and NHS (43.7 mg, 379.7 μM). After each reagent was dissolved, the reaction was stirred overnight at 40°C. The reacted system was fully dialyzed in methanol, methanol / water (1 / 1), pure water (containing 12.5 mg / ml of sodium chloride) and pure water successively, each dialyzing for 12 hours. After sufficient dialysis to remove the organic solvent, freeze-dry to obtain white po...
Embodiment 3
[0103] The preparation method three of HA-CA amphiphilic hyaluronic acid copolymer, the reaction equation is as follows image 3 :
[0104] The hyaluronic acid-5β-cholanic acid prepared by the method shown in Example 1 was dissolved in formamide, EDC was added, reacted at room temperature, and then NHS was added to prepare a reaction solution; then Dissolve in DMF, and slowly drop into the reaction solution prepared above to react to prepare the hyaluronic acid-5β-cholanic acid. The reacted system was fully dialyzed in methanol, methanol / water (1 / 1), pure water (containing 12.5 mg / ml of sodium chloride) and pure water successively, each dialyzing for 12 hours. After sufficient dialysis to remove the organic solvent, freeze-dry to obtain white powder HA-CA, which is stored at low temperature until use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com