Raoultella terrigena for synthesizing 2,5-furan dicarboxylic acid and application of raoultella terrigena
A technology of furandicarboxylic acid and bacteria, which is applied in the field of microorganisms, and achieves the effect of simple method and good application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 The screening of high-yield 2,5-furandicarboxylic acid strains
[0042] Add 1 g of a soil sample taken from a nearby factory producing 5-hydroxymethylfurfural into a 250 mL Erlenmeyer flask containing 20 mL of enrichment medium, and incubate at 30°C and 220 rpm for 24 hours. After the culture solution was centrifuged for a short time, the supernatant was taken and applied to the selection medium for cultivation for 48 hours. Pick the strains with larger colonies and transfer them to the seed medium for 24 hours of cultivation, then transfer 500 μL of the culture solution into a preservation tube containing 500 μL of 40% glycerol, and store in a -80°C refrigerator.
[0043] Enrichment medium or fermentation medium (g / L): tryptone 10g, yeast powder 5g, sodium chloride 10g, 5-hydroxymethylfurfural 3.15g.
[0044] Screening medium (g / L): tryptone 10g, yeast powder 5g, sodium chloride 10g, 5-hydroxymethylfurfural 4.4g, agar 20g;
[0045] Pick the strains stored a...
Embodiment 2
[0049] Example 2 Molecular biological identification of 2,5-furandicarboxylic acid strain
[0050] The 16sDNA sequences were amplified from the genomes of the screened strains using bacterial universal primers (27F / 1492R), sequenced, and compared. The 16sRNA sequence of the strain is shown in SEQ ID NO.1. The sequence was compared in NCBI by Blast, and it was found that the similarity between the target sequence of the bacterial strain and Raoulleia terrigena (R. terrigena NBRC14941) was 98%. Known as Klebsiella terrigena (Klebsiella terrigena)). The strain was deposited in the China Center for Type Culture Collection on August 26, 2014, with the preservation number CCTCC No: M2014391, and the preservation address is Wuhan University, Wuhan, China.
Embodiment 3
[0051] Morphological characteristics and physiological and biochemical characteristics of embodiment 3Raoultella terrigena BF60
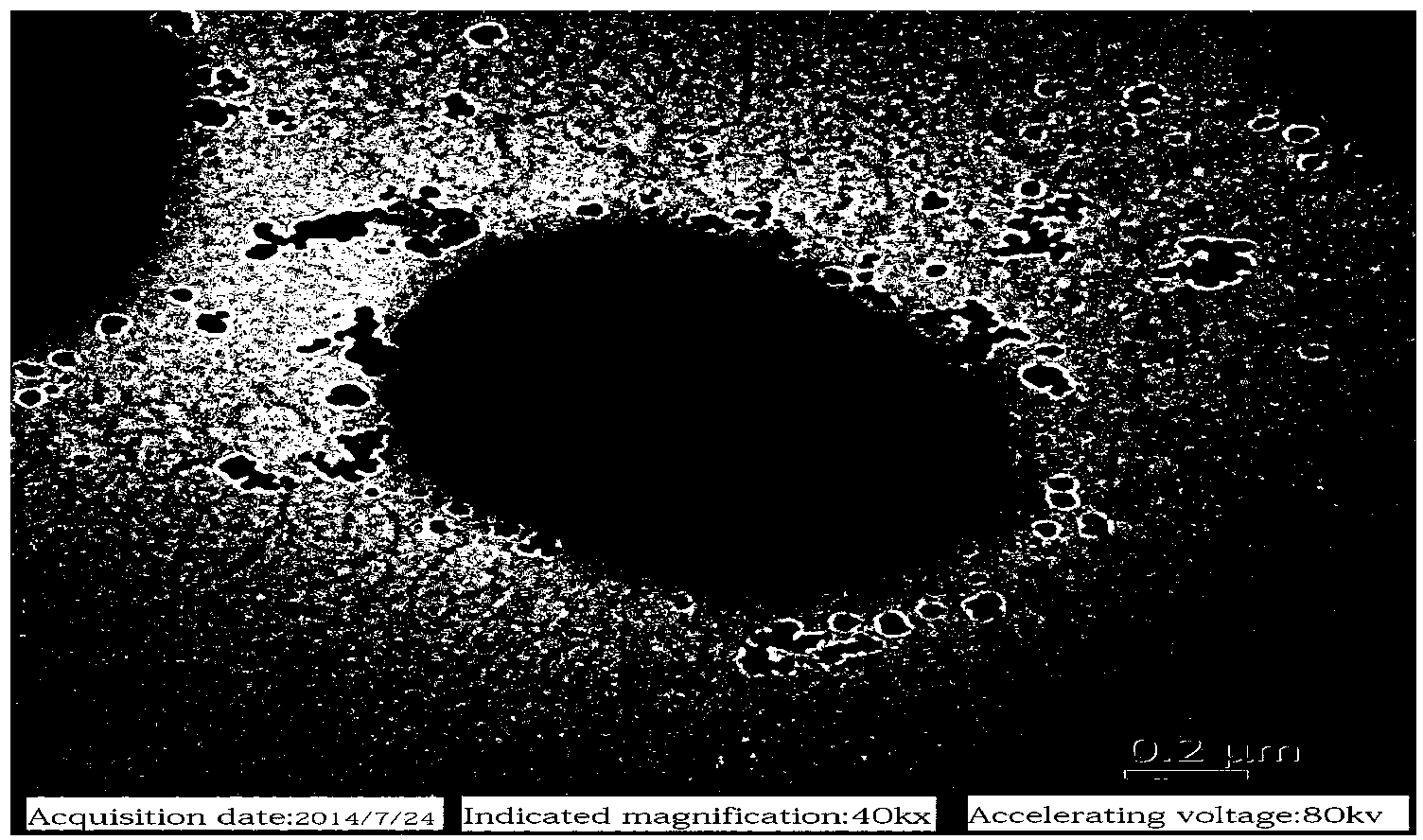
[0052] R. terrigena BF60 was cultured at 30°C for 24 hours on a screening medium without 5-hydroxymethylfurfural, the colony was light yellow hemispherical, the surface was wet and shiny, the edges were neat, translucent, facultative anaerobic growth, Gram Staining was negative, 1000 × microscope (attached figure 1 ) and transmission electron microscopy (attached figure 2 ) observed under the rod-shaped cells, with capsules, single or in pairs. Corresponding physiological and biochemical performance analysis experiments were carried out with reference to the eighth edition of "Berger's Bacterial Identification Handbook" and related literature, and the results are shown in Table 1.
[0053] Table 1 Classification and identification results of target strains
[0054]
[0055] This result is consistent with the characteristics shown by Raoultell...
PUM
| Property | Measurement | Unit |
|---|---|---|
| collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com